Did you know that fishermen sometimes stab fish before releasing it back into the ocean? This practice in releasing fish, called venting, seems odd at first, but the reason lies behind the complexities of a unique organ: the swim bladder. Swim bladders, internal gas-filled organs that control buoyancy, inflate with decreasing external pressure, when the animals are rapidly reeled in by fishermen, which might cause a variety of injuries summarized as barotrauma. When the air inside swim bladders expands, it exerts pressure on other internal organs and may even push fish’s stomachs through their mouths, prohibiting the animals from eating. Another potential issue is the extra buoyancy generated by the enlarged swim bladder, preventing the fish from swimming back down to their habitat depth and leaving them vulnerable to predators. Besides organs containing gas, fluids like blood plasma with dissolved gasses are also affected by changes in pressure. When the external pressure drops, gas escapes from blood to form air bubbles which can continue to expand and rupture organs. Inflated swim bladders are one of the primary causes of post-release fish mortality. A close examination of how swim bladders function in fish can help explore and evaluate potential release strategies, including venting.
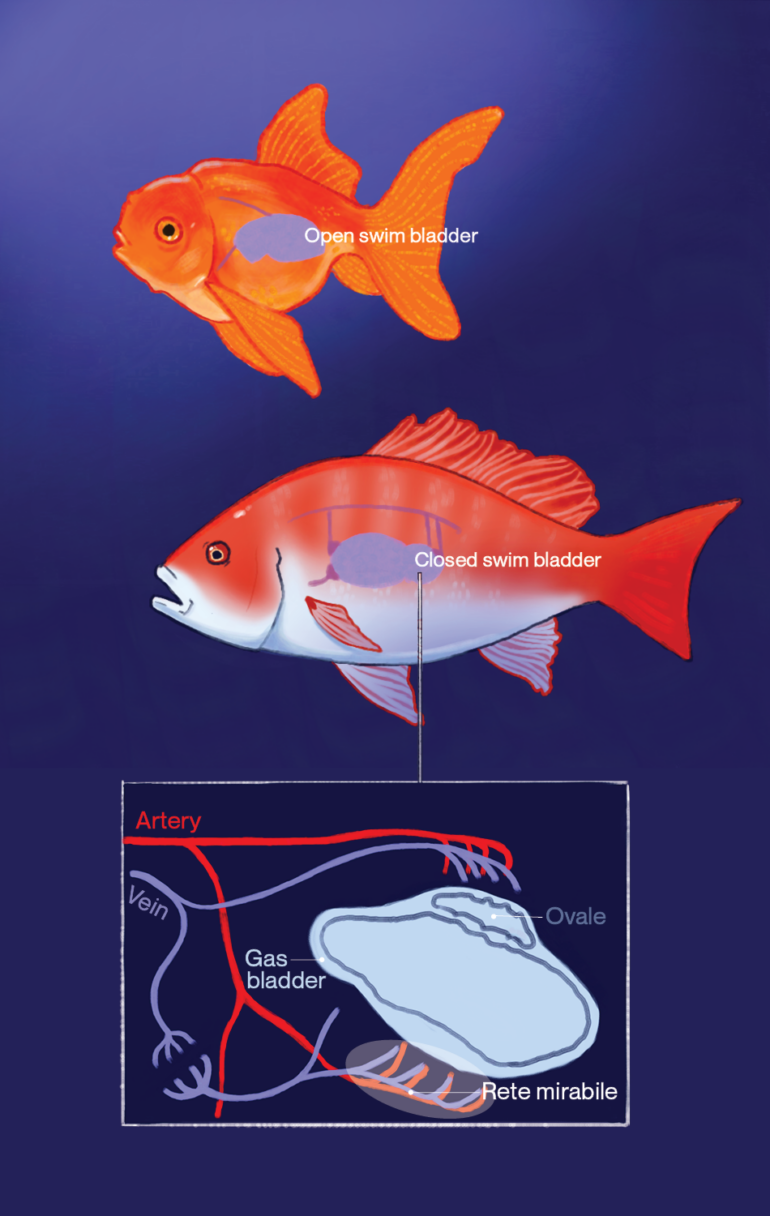
Fish obtain neutral buoyancy by adjusting the size of their swim bladders. The wall of the swim bladder is impermeable to gas, allowing it to regulate the amount of air inside. Through increasing or decreasing the amount of air inside the swim bladders, fish can achieve the same density as the surrounding water. When this happens, the total buoyancy force balances the gravitational force, which allows fish to reach a state of neutral buoyancy so it can spend less energy trying to avoid sinking or rising in the vast ocean. The majority of swim bladders in fish can be classified into two categories: open or closed. Barotrauma affects these two types differently.
-
Open swim bladder
Fish with an open swim bladder are called physostome fish. Some examples include trouts, carps, and catfish. They have a tube that connects the swim bladder to the esophagus. Physostome fish rely on gulping air at the surface of water to inflate their swim bladders. Thus, the gas composition in the swim bladder is similar to air. They can also vent their inflated swim bladders through “belching”—similar to us when we release excessive air from our mouths after eating too fast–to reduce buoyancy when they are on the surface. If they are brought to the surface by the angler, therefore, they can quickly readjust their swim bladders by releasing air through their mouths to alleviate barotrauma.
-
Closed swim bladder
Fish with a closed swim bladder, known as physoclist fish (e.g. rockfish, snappers, sea bass), lack a tube that connects the swim bladder to the esophagus. Instead, it has a gas gland inside that facilitates the diffusion of O2 by creating an acidic environment. Cells in the gas gland produce CO2 and lactic acid from anaerobic and aerobic metabolism, which acidify the adjacent blood vessels. This adjustment in pH then promotes the offloading of O2 from hemoglobin, a protein in the blood cell, through two effects. The Bohr effect states that hemoglobin’s ability to bind to O2 lowers in an acidic environment, which happens as CO2 concentration increases. However, hemoglobin can still become saturated with O2 if the amount of dissolved O2 in the blood is high enough. Secondly, the Root effect describes how acidity reduces cooperativity among subunits in hemoglobin. Hemoglobin is composed of four protein subunits. Usually, the binding of O2 to the first subunit changes the shapes of other subunits to increase their affinity to O2. However, an acidic environment destroys the cooperativity among subunits. In contrast to the Bohr effect, even if O2 is abundant in the blood, hemoglobin cannot be fully saturated based on the Root effect. The combined Bohr and Root effects induced by the addition of CO2 and lactic acid in the gas gland favor the release of O2 from hemoglobin into the swim bladder.
To inflate the bladder, a pressure gradient in the blood is created to facilitate gas exchange. The gas gland is sandwiched between the bladder and the rete mirabile, a network of blood vessels that utilizes a countercurrent system to maximize the exchange of O2. Rete mirabile in Latin translates to “a wonderful net”, which describes the complex interwoven structure of small blood vessels called capillaries. Capillaries connect arteries and veins that carry blood away and to the heart respectively. Arterial capillaries with a higher partial pressure of oxygen (PO2) and venous capillaries with lower PO2 sit next to each other, but flow in opposite directions, constructing a countercurrent exchange system. This system maintains a high PO2 in the touching phase where O2 can readily diffuse from the capillaries, through the gas gland, into the swim bladder.
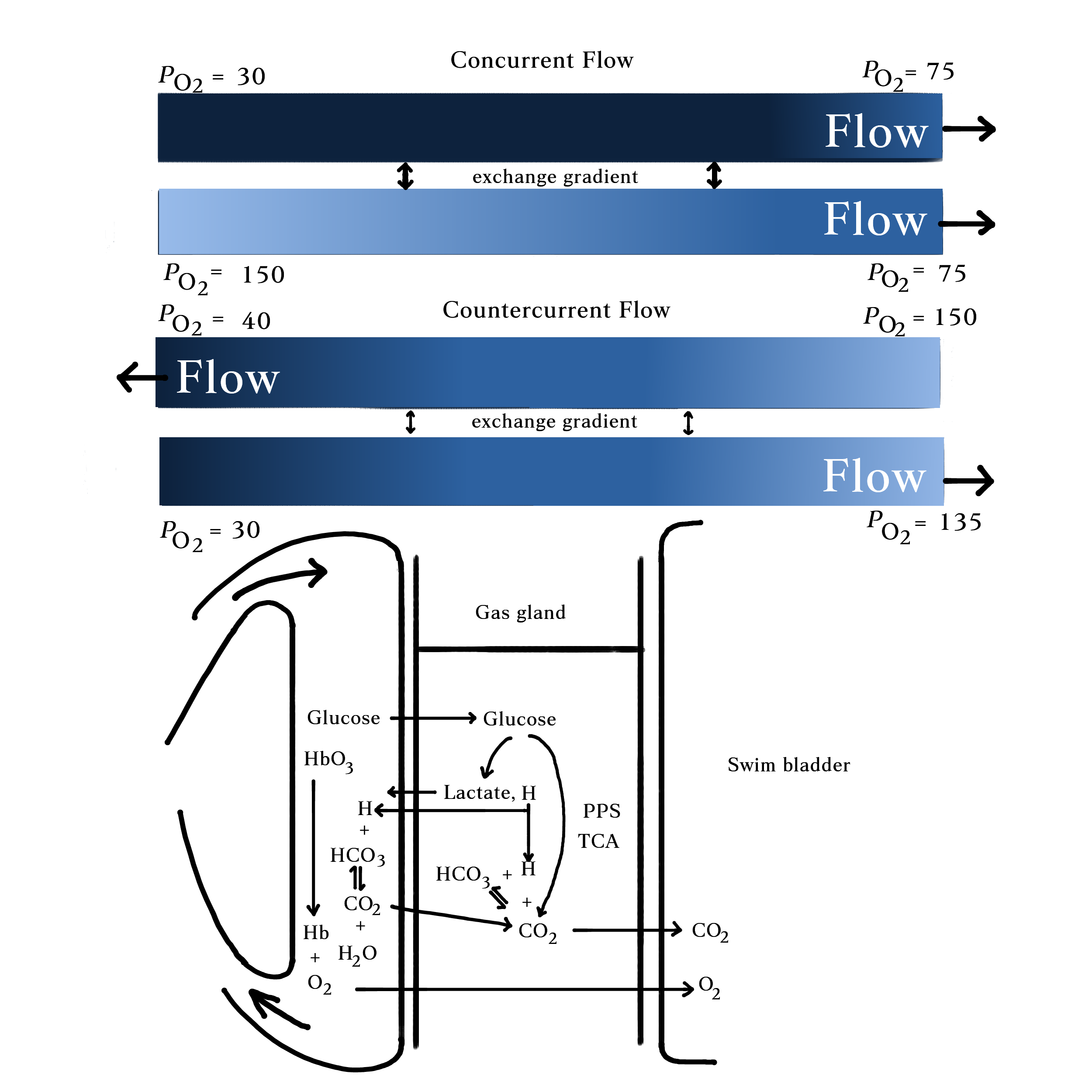
When the gas has to escape, it goes through a secretory region–the oval. The oval is connected to abundant blood vessels and has a muscular valve. Under neuronal and hormonal control, the oval can contract or relax the muscle to allow reabsorption of gas into the blood. Since the partial pressure of gas in the swim bladder is higher than in the blood due to concentrated oxygen, gas can readily diffuse into the blood vessels when the oval opens. However, this process still takes much longer than “burping” the air through the mouth in an open swim bladder, so physoclist fish with closed swim bladders are reported to be more susceptible to barotrauma than physostome fish.
-
Barotrauma and Venting
In both recreational and commercial fisheries, it is a common practice to return live fish back into the water because of their low economic value or to comply with fishery management regulations. Often, to increase the survival rate of post-release fish, fishermen vent or stab a fish with a specialized hollow hypodermic needle to deflate its expanded swim bladder. Naturally, many studies have examined the effectiveness of this release strategy. In a 2011 report, Dr. Gene R. Wilde analyzed data from 39 samples in 17 studies to compare the survival rate of vented and unvented fish. He concluded that this technique should be discouraged, since venting only slightly increased the survival rate of fish caught from shallow water, and was even detrimental to fish caught from deep water. Furthermore, he pointed out that the experimental design of many of these studies were flawed because the rate of survival of unvented fish was usually excluded, failing to demonstrate that venting contributed to a high survival rate. In contrast, Drumhiller et al. utilized chambers that can simulate the water pressure of different depths to investigate the recovery rate of red snappers from barotrauma after rapid recompression and venting in a 2014 study. Their results suggested that venting and recompression are effective tools to reduce fish discard mortality. Both studies shed light on proper usage of this venting technique, yet its effectiveness remains fiercely debated.
Advancements in technology constantly deepen our understanding of the swim bladder. In addition to regulating buoyancy, the swim bladder is now considered to play a role in other mechanisms such as sound detection and respiration. As technology also promotes the expansion of industrial and recreational fisheries to meet our demand for fish, the fishing industry must consider their role in fishing responsibly. While they make considerable effort into catching more fish, less attention is paid to the issue of releasing fish while ensuring their best chances at recovery. Mortality in released fish is an ongoing issue that results in significant ecosystem degradation. When we prioritize the development of techniques for effective exploitation of natural resources, we should also consider the sacrifices to achieve those “desirable” results.
Resources:
- Berenbrink, Michael, et al. “Evolution of oxygen secretion in fishes and the emergence of a complex physiological system.” Science 307.5716 (2005): 1752-1757.
- Bohr, C., K. Hasselbalch, and A. Krogh. “Concerning a biologically important relationship–the influence of the carbon dioxide content of blood on its oxygen binding.” Skand. Arch. Physiol 16 (1904): 401-412.
- Pelster, Bernd. “5 Buoyancy At Depth.” Fish physiology. Vol. 16. Academic Press, 1997. 195-237.
- Watanabe, Y., et al. “Swimming behavior in relation to buoyancy in an open swimbladder fish, the Chinese sturgeon.” Journal of Zoology 275.4 (2008): 381-390.
- Rudershausen, P. J., B. J. Runde, and J. A. Buckel. “Effectiveness of venting and descender devices at increasing rates of postrelease survival of Black Sea Bass.” North American Journal of Fisheries Management 40.1 (2020): 125-132.
- Wilde, Gene R. “Does venting promote survival of released fish?.” Fisheries 34.1 (2009): 20-28.
- Drumhiller, Karen L., et al. “Venting or rapid recompression increase survival and improve recovery of Red Snapper with barotrauma.” Marine and Coastal Fisheries 6.1 (2014): 190-199.
- Pelster, Bernd, and Peter Scheid. “Countercurrent concentration and gas secretion in the fish swim bladder.” Physiological zoology 65.1 (1992): 1-16.
- Ross, L. G. “The haemodynamics of gas resorption from the physoclist swimbladder: the structure and morphometrics of the oval in Pollachim virens (L).” Journal of Fish Biology 14.3 (1979): 261-266.
- Brown, Richard S., et al. “Understanding barotrauma in fish passing hydro structures: a global strategy for sustainable development of water resources.” Fisheries 39.3 (2014): 108-122.
- Skoglund, C. R. “Functional analysis of swim-bladder muscles engaged in sound production of the toadfish.” The Journal of Cell Biology 10.4 (1961): 187-200.