Do you remember the first time you rode a bike? For many, tightly grasping the handlebars and swerving from side to side in a desperate attempt to stay in balance is but a distant memory. Now, whether cruising down the sidewalk or rushing to make it on time to your next class, biking has become a movement almost as familiar as walking. Motor learning is the process through which skills involving movement are acquired. With practice and repetition, repeated movements are refined and committed to memory and like riding a bike, can be later performed effortlessly. On the neuronal level, however, learning and memory are processes involving a dynamic neural network where neural connections are formed and eliminated.
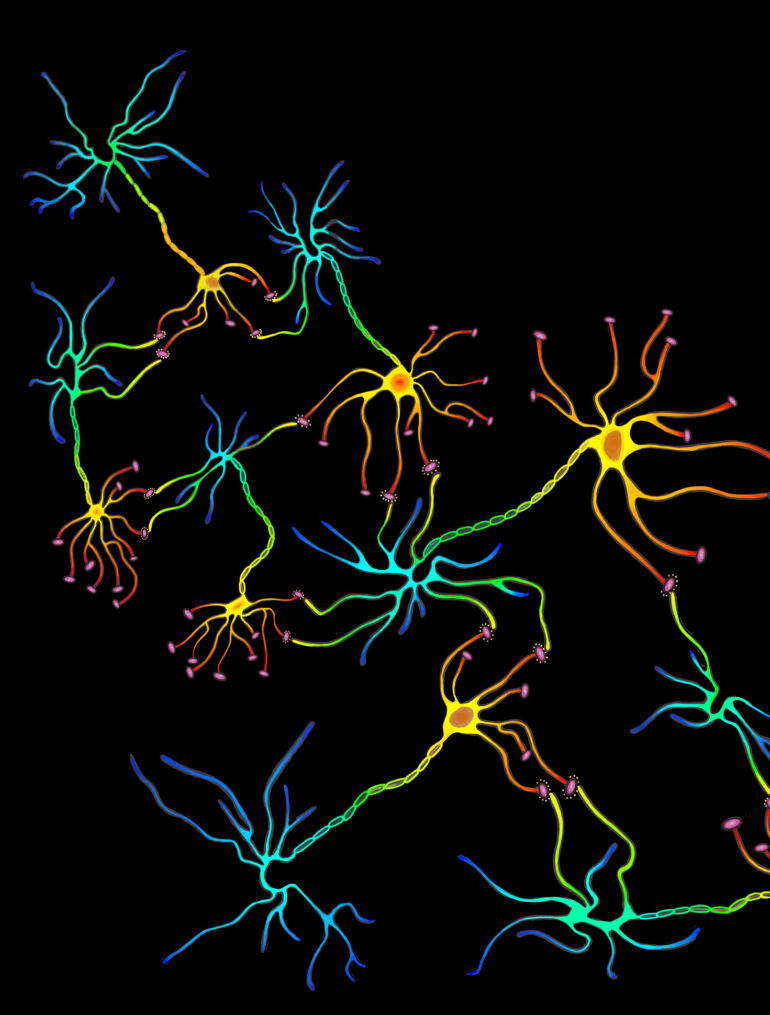
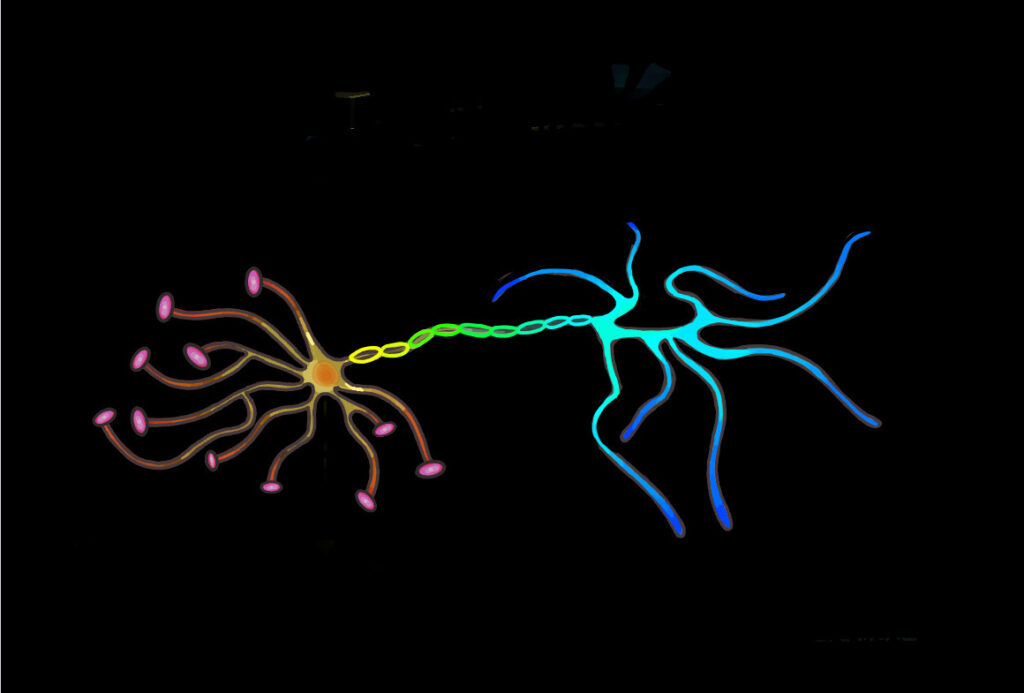
The neural network consists of neurons and the synaptic connections formed between them. These connections are predominantly formed between the axonal bouton, a bulbous growth located on the axon terminal of a presynaptic neuron, and the dendritic spine of a postsynaptic neuron. Dendritic spines are protrusions found on the dendrites of neurons and contain excitatory synapses through which neurotransmitters can be received to allow for the propagation of electrical signals across neural circuits. Spines are dynamic and can change in response to stimuli. This, in turn, alters the area of contact between the presynaptic axon terminal and postsynaptic dendrite and influences the capacity for transmission between neurons, strengthening or weakening synaptic connections. The summation of this process shapes and forms developing circuits, leading to alterations in the neural network. A distinct correlation between changes in spine morphology and population have been observed in response to learning. As a result, researchers are interested in closely examining dendritic spine dynamics in various learning paradigms, including that of motor learning.
To investigate motor learning, researchers must first induce the learning process to then observe any changes in the physiology of the nervous system. A well-established method is the use of animal models, such as the mouse. A study published by Xu et al. in 2009 trained mice in a forelimb reaching task and demonstrated the rate of dendritic spine formation to increase following novel motor skill learning. The task required the mice to reach between an opening with a single forelimb for a food reward. The study found that new connections form specifically on the side of the motor cortex opposite to the reaching forelimb as rapidly as within an hour of training initiation. The study further demonstrated a linear correlation between the number of successful reaches performed during the first training session and the percentage of spines formed following the session, indicating a strong association between learning and the formation of new spines. An increase in spine formation, however, was not observed in mice tasked to perform an easier version of the task, where the food reward was placed within reach of both paws instead of a single forelimb, nor when presented with a target beyond their reach. This further suggests spine formation occurs with the refinement of fine motor movements as opposed to general motor activity.
Counterintuitively, though novel motor tasks drive increased dendritic spine formation, dendritic spines are also eliminated with prolonged learning, returning the total spine density in the area to its initial amount. Preexisting spines present prior to the introduction of the novel motor task are preferentially eliminated while stabilized, newly-formed spines are preserved. Repetition of the motor task leads to the persistence of selected novel spines beyond the duration of the training period and suggests the consolidation of lasting motor memories. Hence, learning can be described as the rewiring of the neural circuit as opposed to simply increasing the number of spines present. As spine elimination refines neural networks and contributes to the plasticity of neuronal connections, the process is crucial for further learning to take place. Impediment to the spine elimination process is associated with intellectual disability as well as behavioral and cognitive impairment, such as diseases including schizophrenia and Alzheimer’s.
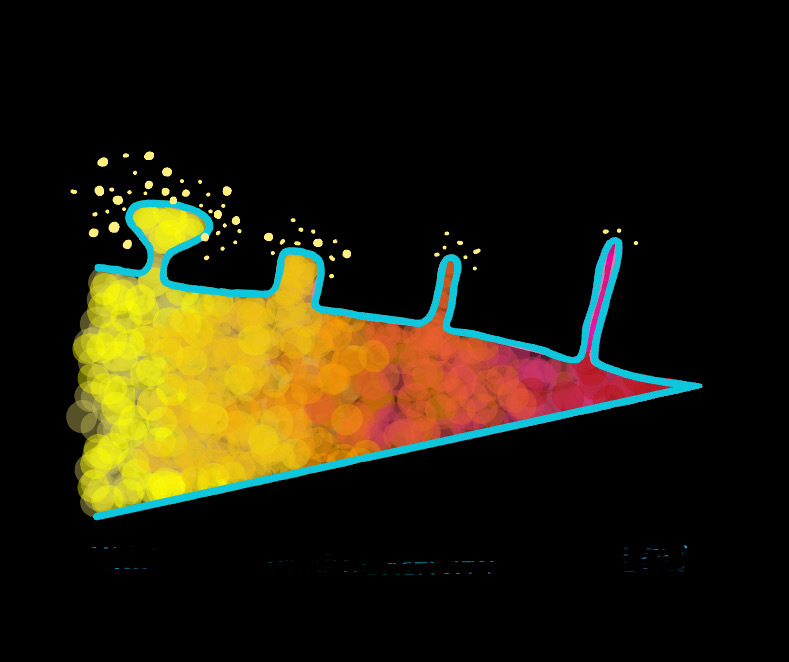
Changes in spine morphology are also indicators of on-going learning and memory consolidation. Two notable categories are thin and mushroom spines, which are described as learning and memory spines respectively. Mushroom spines, characterized by large bulbous heads and narrow necks, are believed to be mature spines. Speculated to be incapable of increasing in synapse size or strength due to their large size, further synaptic strengthening of mushroom spines is limited. Hence, mushroom spines may indicate consolidated memory. Thin spines, on the other hand, could indicate synaptic plasticity and the capacity for learning. Small and immature, these spines are capable of greater synaptic strengthening. Thin spine count has also been found to decrease with aging and cognitive deterioration which suggests these spines to be associated with learning.
Dendritic spine dynamics regulate and refine the neural network, promoting the plasticity of neural circuits which make motor learning and memory possible. As our understanding of learning and memory consolidation deepens, questions start to arise about whether memory can be manipulated. Given that we have identified a physical neurobiological basis for memory in the form of dendritic spines, could we manipulate memory on the synaptic level through targeting these very structures? Dendritic spine dynamics and its role in learning and memory presents a compelling direction of study for both neurobiological understanding and biomedical uses alike. As the neural network and its organization as a mesh of interconnected units become increasingly unraveled, perhaps the role of dendritic spines in the complex processes of learning and memory may become clearer.
Works Cited:
www.sciencedirect.com/science/article/pii/S1084952121001245
www.nature.com/articles/nature08389