By Stephen Calderon | UTS Staff Writer | SQ Online (2015-16)
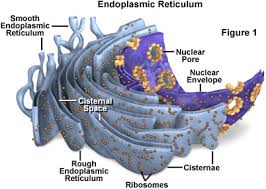
At any given moment, our cells are under fire from a variety of negative stimuli and face the challenge of constantly maintaining homeostasis. Our cells have evolved clever and efficient ways of counteracting these stimuli. One of the cell’s most important organelles, the endoplasmic reticulum (ER), functions to fold newly synthesized proteins. Problems arise when factors inhibit the protein folding machinery of the ER, leading to the accumulation of unfolded proteins in the ER lumen. This phenomenon is called ER stress which can be fatal to cells if left unchecked and has become a subject of current medical research at UC San Diego. Our cells have evolved an adaptive response to ER stress called the unfolded protein response (UPR) to help restore the balance. The UPR consists of signaling pathways that elicit responses that could either keep the cell alive or induce apoptosis. Its direct influence on cell survival has been linked to medical conditions, such as diabetes, Alzheimer’s, and cancer. Led by Dr. Maurizio Zanetti, researchers at the UCSD Laboratory of Immunology have devoted their efforts to studying the UPR’s association with cancer cells and their exploitation of the response to promote tumor growth and protection.
Unraveling the UPR
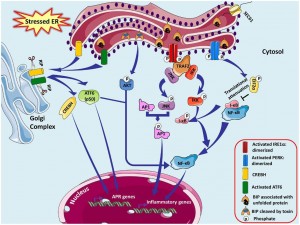
The UPR is a defense mechanism against a wide range of stimuli that result in accumulation of unfolded proteins in the lumen, such as nutrient deprivation, low oxygen supply, and harmful reactive intermediates. Once unfolded proteins and subsequent ER stress begin to increase in the cell, it is sensed by IREα, PERK and ATF6, a group of signaling molecules that activate pathways to normalize protein folding. PERK specifically inhibits protein translation while IREα and ATF-6 increase the production of ER chaperone molecules that assist in protein folding. This reduces excess protein accumulation in the ER lumen.
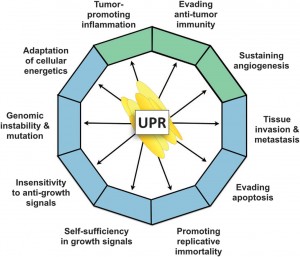
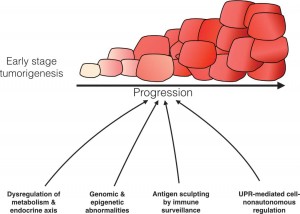
Over the last few years, many different types of cancer cells have shown high expressions of key UPR pathway proteins, suggesting that UPR signaling plays an important role in cancer biology. Studies involving over- expressing or knocking out these genes revealed that both modifications had strong influences on tumor formation and longevity, strengthening speculation that UPR-based signaling has a significant effect on processes linked to tumorigenesis. The results of these experiments suggest that the tumor itself is initiating a UPR-based system to improve its ability to survive and proliferate.
Further research also shows that UPR signaling significantly influences many immunological aspects of the tumor and its surrounding microenvironment. The tumor UPR genes (IREα, PERK, ATF6) have shown to regulate processes involved in inflammation that favor the transcription of well-known inflammatory genes. Inflammation is a immune response our body uses to clear up harmful stimuli or pathogens in the case of injury or infection. Downstream signals of these molecules are also responsible for the expression of pro-inflammatory cytokines as well which are part of a broad class of secreted molecules that play active roles in immune response. Since inflammation is a powerful tool for recruiting immune cells to the tumor environment, it is highly beneficial to the tumor. These cells then secrete nutrients and cytokines that can jumpstart other pro-tumor processes. UPR signaling has also been shown to play a significant role in regulating angiogenesis, the process by which new blood vessels are formed. At the same time, UPR-based mechanisms affect the immune cells themselves by making these cells less efficient at being able to recognize foreign invaders.
The impact of the Zanetti Laboratory on ER stress
Why are our bodies unable to simply fight cancer off like any other infection? Keeping in mind the role UPR-based signaling in antitumor immunity, it was discovered in Dr. Zanetti’s laboratory that cancer cells are actually able to induce ER stress onto certain cell types. This is accomplished by an unidentified secreted factor(s) that targets cells such as the precursors to the macrophage white blood cells called myeloid cells. This induced ER stress response was found to cause pro-inflammatory, pro-angiogenic, and immunosuppressive phenotypes in the receiving cells. A byproduct of this reaction also causes indirect decrease of certain T cells. This is an attempt by the tumor to not only ensure its survival, but also to diminish the body’s immune response against it.
With this newfound information, Dr. Zanetti and his team are currently investigating the role of ER stress transmission on both cancer cells and receiver myeloid cells in addition to looking at the signaling pathways that are consequently activated. Another goal of the Zanetti laboratory is to chemically elucidate the factor(s) involved with ER stress transmission. This is important since the factor(s) are believed to not only transmit ER stress, but also play an active role in inhibiting the immunological capabilities of the myeloid cells that normally would aid in tumor control.
What does this mean for of cancer treatments?
Much more work is needed to understand these complicated processes, but what can be said is that ER stress and the UPR present a new possible angle on which medicine may get a foothold on cancer. It is through this kind of research that laboratories at UCSD are paving the way for medical breakthroughs. For example, if the research at the Zanetti lab continues to characterize these pathways and identifies some of the individual factors involved, they may discover new targets for medical treatments. Tumors have a tendency to reappear despite chemotherapy and radiation treatment. Based on increasing body of evidence, it is clear that the tumor is able to utilize important pathways to protect itself and spread. Therefore, treatments aimed at inhibiting the tumor’s defense mechanisms may be successful countermeasures against cancer.
[hr gap=”0″]
Sources:
- http://www.ncbi.nlm.nih.gov/pubmed/8898193
- http://www.ncbi.nlm.nih.gov/pubmed/18039139
- http://www.ncbi.nlm.nih.gov/pubmed/21266244
- http://scienceblog.cancerresearchuk.org/2013/02/01/feeling-the-heat-the-link-between-inflammation-and-cancer/
- http://www.ibiology.org/ibiomagazine/issue-1/peter-walter-unfolding-the-upr.html
- http://www.nature.com/onc/journal/vaop/ncurrent/full/onc2015108a.html