INTRODUCTION
Obsessive-Compulsive Disorder (OCD) is a psychiatric illness characterized by intrusive thoughts, designated as obsessions, and repetitive compulsions, such as motor-related fixations.¹ Symptoms of OCD include checking behavior–compulsive interactions with switches or locks–and fixation on symmetry, hygiene, or routine tasks. Standard clinical approaches to OCD are cognitive behavioral therapy (CBT) and treatment with selective serotonin-reuptake inhibitors (SSRIs).¹ CBT aims to reduce anxiety by retraining responses to unwanted obsessional thoughts, replacing checking behavior with safer interactions, for example; this psychiatric approach is routinely supplemented with SSRIs, which increase extracellular concentrations of serotonin via inhibition of the serotonin (5-HT) transporter. However, roughly forty to sixty percent of patients diagnosed with OCD are inadequately treated with this clinical approach.¹ OCD patients also experience a high rate of comorbidity with bipolar disorder (BP – OCD), among other conditions, which further complicates treatment with the standard CBT-SSRI approach.
Dysfunction of the cortico-striato-thalamo-cortical (CSTC) pathway loops is implicated in the emergence of OCD symptoms.² This pathway is primarily responsible for regulation of reward processing, executive motor function, and habit formation; it is also targeted by SSRIs and other alternative treatments.
Treatment-resistant patients typically repeat SSRI trials with increased potency or dosage. Continued treatment failure will lead clinicians to prescribe alternative pharmacotherapies; the mechanisms of action of these treatments range widely, from dopamine agonism to serotonin agonism to glutamate antagonism, visualized in Figure 1. Each of these pharmacotherapies act selectively on their target receptor, yet each produces symptom relief. By studying changes in neurotransmitter signaling in patients and the response to each of these treatments, this paper will review alternative therapies and what their mechanisms of action suggest about the physiology of CSTC pathway loops.
DOPAMINE SIGNALING IMPLICATED IN OCD MODEL REWARD PROCESSING AND COMPULSIONS
Treatment with the dopamine agonist aripiprazole in addition to SSRI/CBT therapy produces relief of OCD symptoms in a general OCD patient group; aripiprazole also displays high potency for striatal D2 Rs.³, In patients experiencing comorbidity with bipolar disorder, co-administration of D2 R agonists and SSRIs was especially effective in alleviating high-risk symptoms, primarily suicidal ideations.
Murray and colleagues investigated anterior cingulate cortex (ACC) and nucleus accumbens (NAc) dopamine signaling in OCD patients and a healthy volunteer group treated with the dopamine antagonist amisulpride and agonist pramipexole, which selectively bind D2 and D3 dopamine receptors (D2/3R), in order to study impaired dopamine signaling in patients. They found that both D2/3R agonism and D2/3R antagonism partially reduced error during prediction of reward, which is typically exacerbated in OCD patients. This paper suggests that this “bidirectional” effect may be due to other neurotransmitter circuits being implicated as a result of impaired dopamine signaling.
In mice, administration of a D2/3R agonist increased compulsive checking behavior, while D2/3R antagonism reduced checking.â· Compulsive behavior under extended D2/3R agonism was also associated with reduced D2R expression in the dorsal striatum. Perani et al. further demonstrates, with PET imaging, a reduction in D2 receptor availability in the ventral striatum of OCD patients; impaired D2 R activity throughout the basal ganglia is also observed to occur alongside decreased 5-HT2 R expression throughout the frontal cortex, cingulate gyrus, and parietal cortex, suggesting strong cross-regulation of CSTC dopamine and serotonin circuitry between these regions.â¸
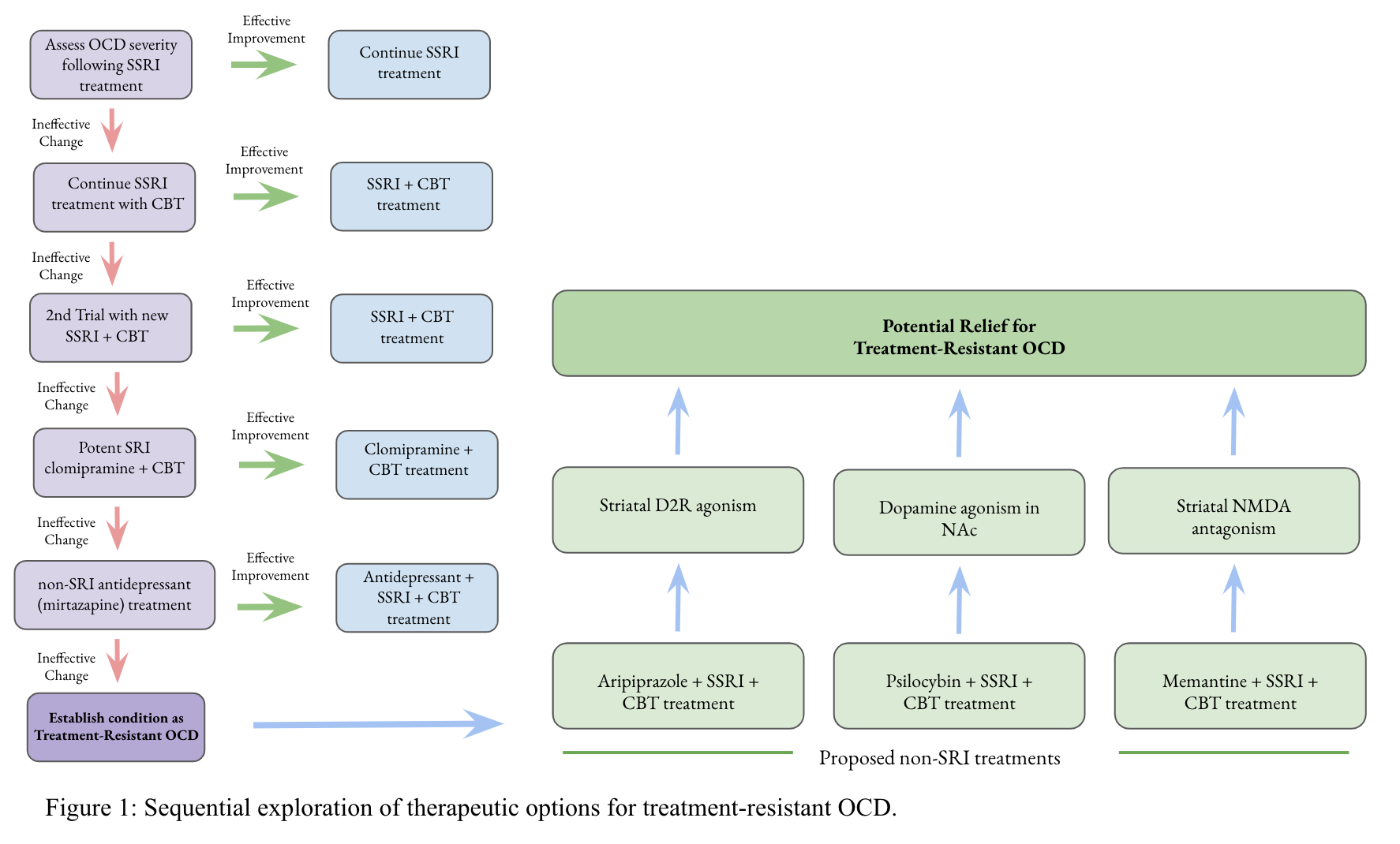
Sequential exploration of therapeutic options for treatment-resilient OCD.
SELECTIVE SEROTONIN SIGNALING ELICITS OBSESSIVE-COMPULSIVE (OC) SYMPTOMS
Serotonin-2 (5-HT2), 5-HT1B, and 5-HT1D receptors have all been implicated in OCD. Clomipramine and fluoxetine, a tricyclic antidepressant and SSRI, respectively, are typically used for treatment, and in mice these drugs downregulate 5-HT1BR signaling in the orbitofrontal cortex (OFC), relieving OCD symptoms.â¹ OFC 5-HT1BR activation is shown to be both sufficient and necessary for the emergence of symptoms.9 Researchers also observed that an agonist that selectively binds 5-HT1D receptors, sumatriptan, heightens symptoms, while 5-HT1AR agonism failed to produce the same effect.¹â° This implicates 5-HT1DR hyperactivity as an additional pathology for OCD; high 5-HT1DR populations in the substantia nigra may play a role in dysregulation of CSTC circuitry.
Reduced 5-HT2 R binding in the frontal and cingulate cortex of OCD patients, as demonstrated by Perani et al, supports the hypothesis that SSRI treatment is mediated by attenuating hypoactivity of cortical 5-HT2 R expression.⸠The mechanism of action for SSRI treatment is not fully understood, but it may partially function by restoring 5-HT2C signaling; in animal studies, a selective 5-HT2C antagonist potently reverses the treatment effects of the SSRI fluoxetine.¹¹ Reduced 5-HT2 R expression also occurs alongside increased 5-HT1 R expression in OCD patients, further speaking to the complicated physiology of CSTC circuits.
The indolamine psilocybin is a low-selectivity serotonin agonist that increases prefrontal cortex (PFC) serotonin signaling and CSTC 5-HT2 R expression, while decreasing PFC dopamine signaling.¹² Administration of low doses of psilocybin in a small sample study reduced severity of OCD symptoms in patients.¹³ SSRI drugs typically take a few weeks to produce relief, but psilocybin produces faster increases in 5HT2AR occupancy with high-potency binding; the strength of this serotonergic agonist may produce relief in patients insufficiently treated with SSRIs.¹
GLUTAMATE DYSREGULATION AFFECTS CORTICOSTRIATAL SIGNALING
Expression of a genetic variant of glutamatergic gene SCL1A1 is hExpression of a genetic variant of glutamatergic gene SCL1A1 is heightened in the population of patients with treatment-resistant OCD, pushing scientists to investigate the implications of impaired glutamate signaling. SCL1A1 encodes the postsynaptic glutamate transporter EAAT3; in the brain, EAAT3 inhibits NMDA receptor (NMDAR) response and disruption of EAAT3 produces stronger NMDAR activation.15 In mice with OCD-like behaviors, corticostriatal synapses showed increased NMDAR activity, as well as increased mGluR5 activity.¹
Administration of NMDAR antagonists, like memantine, also helps alleviate treatment-resistant conditions. Memantine was successful in double-blind treatment-resistant OCD trials, showing much greater efficacy for the treatment-resistant group versus the treatment-compliant group.17 Memantine co-treatment with SSRIs is a strong strategy for treating patients expressing variants of glutamatergic genes, particularly SCLA1, since SSRIs alone fail to mediate glutamate hyperactivity in CSTC circuits.
CONCLUSION
Each potential therapy demonstrates aspects of improvement over SSRI-CBT treatment alone. Aripiprazole treatment shows great potential for treating high-risk OCD symptoms and comorbidity. Administration of psilocybin can serve as a fast-acting alternative to SSRI treatment with increased potency. Memantine shows the most promise in treatment-resistant conditions, by correcting additional pathologies and attenuating SCL1A1-associated hyperglutamatergic activity. Further study into CSTC signaling changes in OCD patients after drug treatment are warranted to better understand the role of these novel pharmacotherapies; as a number of these studies demonstrate, serotonin, dopamine, and glutamate circuits are closely interconnected in the CSTC pathway, and altering expression of any of these neurotransmitters can cause OCD symptoms. Corresponding research may also elucidate which factors are associated with resistance to treatment in OCD patients, potentially leading to a restructuring of the current clinical approach.
REFERENCES
- Wilhelm, S., Tolin, D., and Steketee, G. “Challenges in Treating Obsessive-Compulsive Disorder: Introduction.” Journal of Clinical Psychology 60, 1127-1132 (2004). https://pubmed.ncbi.nlm.nih.gov/15389623/
- Calzà , J. et al. “Altered Cortico-Striatal Functional Connectivity During Resting State in Obsessive-Compulsive Disorder.” Frontiers in Psychiatry vol. 10 (2019). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6524661/
- Muscatello, M et al. “Effect of Aripiprazole Augmentation of Serotonin Reuptake Inhibitors or Clomipramine in Treatment-Resistant Obsessive-Compulsive Disorder: a Double-Blind, Placebo-Controlled Study.” Journal of Clinical Psychopharmacology 134, 174-179 (2014). https://doi.org/10.3410/f.718508460.793497753.
- Mamo, M.D. et al. “Differential Effects of Aripiprazole on D 2 , 5-HT 2 , and 5-HT 1A Receptor Occupancy in Patients With Schizophrenia: A Triple Tracer PET Study.” American Journal of Psychiatry 164, 1411-1417 (2007). https://ajp.psychiatryonline.org/doi/full/10.1176/appi.ajp.2007.06091479.
- Lai, J. et al. “Aripiprazole Augmentation in Managing Comorbid Obsessive-Compulsive Disorder and Bipolar Disorder: a Case with Suicidal Attempts.” Neuropsychiatric Disease and Treatment 13, 87-90 (2017). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5207469/.
- Murray, G. et al. “Dopaminergic drug treatment remediates exaggerated cingulate prediction error responses in obsessive-compulsive disorder.” Psychopharmacology 236, 2325-2336 (2019). https://pubmed.ncbi.nlm.nih.gov/31201476/
- Eagle, D. et al. “The Dopamine D2/D3 Receptor Agonist Quinpirole Increases Checking-like Behaviour in an Operant Observing Response Task with Uncertain Reinforcement: A Novel Possible Model of OCD.” Behavioural Brain Research 264, 207-229 (2014). https://www.sciencedirect.com/science/article/pii/S0166432813007894.
- Perani, D. et al. “In Vivo PET Study of 5HT2A Serotonin and D2 Dopamine Dysfunction in Drug-Naive Obsessive-Compulsive Disorder.” NeuroImage 42, 306-314 (2008). https://www.sciencedirect.com/science/article/abs/pii/S1053811908005041?via%3Dihub.
- Shanahan, N. A., Velez, L. P., Masten, V. L., and Dulawa, S. C. “Essential Role for Orbitofrontal Serotonin 1B Receptors in Obsessive-Compulsive Disorder-like Behavior and Serotonin Reuptake Inhibitor Response in Mice.” Biological Psychiatry 70, 1039-1048 (2011). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3310222/.
- Aouizerate, B. et al. “Updated overview of the putative role of the serotoninergic system in obsessive-compulsive disorder.” Neuropsychiatric Disease and Treatment vol. 1, 231-243 (2005). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2416754/.
- Rodriguez, M. et al. “Behavioral Effects of a Novel Benzofuranyl-Piperazine Serotonin-2C Receptor Agonist Suggest a Potential Therapeutic Application in the Treatment of Obsessive—Compulsive Disorder.” Frontiers 22 (2017). https://www.frontiersin.org/articles/10.3389/fpsyt.2017.00089/full.
- Sakashita, Y. et al. “Effect of Psilocin on Extracellular Dopamine and Serotonin Levels in the Mesoaccumbens and Mesocortical Pathway in Awake Rats.” Biological and Pharmaceutical Bulletin 38, 134-138 (2015). https://pubmed.ncbi.nlm.nih.gov/25342005/
- Moreno, F. A., Wiegand, C.B., Taitano, E. K., and Delgado, P. L. “Safety, Tolerability, and Efficacy of Psilocybin in 9 Patients With Obsessive-Compulsive Disorder.” The Journal of Clinical Psychiatry 67, 1735-1740 (2006). https://doi.org/10.4088/jcp.v67n1110
- Madsen, M.K. et al. “Psychedelic Effects of Psilocybin Correlate with Serotonin 2A Receptor Occupancy and Plasma Psilocin Levels.” Neuropsychopharmacology 48,1328-1334 (2019). https://www.nature.com/articles/s41386-019-0324-9.
- Li, M.H. et al. “Amphetamine and Methamphetamine Increase NMDARGluN2B Synaptic Currents in Midbrain Dopamine Neurons.” Neuropsychopharmacology 42, 1539-1547 (2017). https://www.nature.com/articles/npp2016278
- Escobar, A. P., Wendland, J. R., Chávez, A. E., and Moya, P. R. “The Neuronal Glutamate Transporter EAAT3 in Obsessive-Compulsive Disorder.” Frontiers 10 (2019). https://www.frontiersin.org/articles/10.3389/fphar.2019.01362/full
- Ghaleiha, A., et al. “Memantine Add-on in Moderate to Severe Obsessive-Compulsive Disorder: Randomized Double-Blind Placebo-Controlled Study.” Journal of Psychiatric Research 47, 175-180 (2012). https://www.sciencedirect.com/science/article/abs/pii/S0022395612002956?via%3Dihub.
WRITTEN BY DHILIP RAMAN
Roger Revelle College, B.S. Physiology and Neuroscience, UC San Diego 2021
FROM SALTMAN QUARTERLY VOL. 18
To read the original version, please click here. To read the full version on our website, please click here. To read more individual articles, please click here.