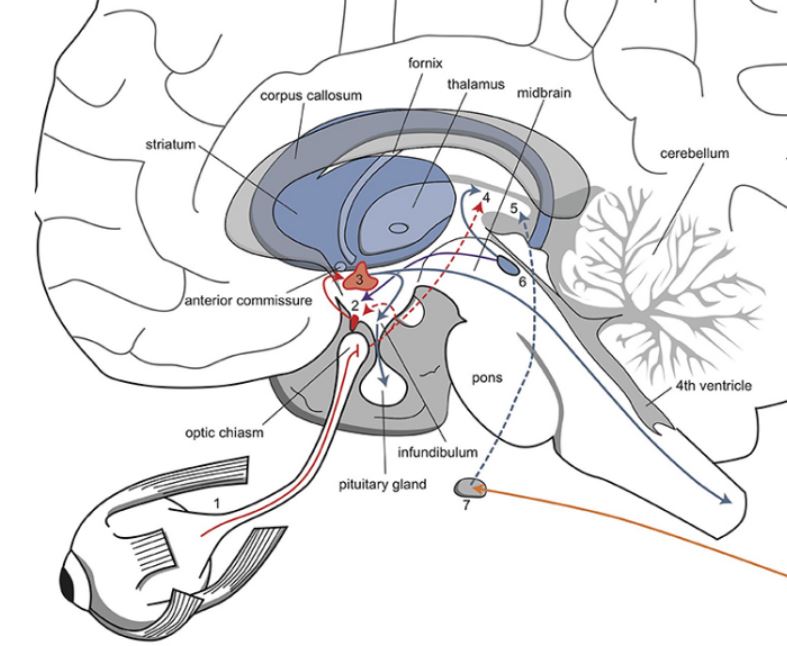
In the figure above, the SCN lies in the shaded region above the optic chiasm, where the left and right optic nerves cross over.
CIRCADIAN RHYTHM 101
The circadian clock is in the suprachiasmatic nucleus (SCN) of the brain.¹ This cluster of nuclei sits atop the optic chiasm, and it receives light input from the retina to time activities such as melatonin release from the pineal gland. Since a rise in melatonin causes sleepiness, the circadian regulation of melatonin regulates sleep. The clock can entrain–or adjust–to different time zones so it does not go out of sync.
Surprisingly, in addition to the SCN, there are circadian molecular clocks all over the body. But what exactly comprises the clock?
INTRODUCTION
Looking closely at natural phenomena, one realizes a common theme–rhythms. The Earth rotates on its axis, revolving around the sun, and the sun in turn rotates on its own axis and revolves around the milky way galaxy. Daily rhythms are vital to helping organisms prepare for the day as well as regulate important functions. These are known as Circadian Rhythms (Latin: Circa-diem, about a day). Given that bodily functions are governed by biochemical reactions, the circadian rhythm controls the timely release of a plethora of chemical compounds. This review seeks to establish the importance of timing in corticosteroid administration to treat autoimmune diseases, due to the mutual dependence between circadian rhythms and glucocorticoids. In mammals, the clock is really a synchronized oscillation in levels of the molecules cryptochrome (CRY) and period (PER). In most mammalian cells, the CLOCK:BMAL1 heterodimer serves as a transcription factor, upregulating the transcription of PER and CRY. During the day, transcription of PER and CRY proceeds until dusk, when the protein products begin to suppress CLOCK:BMAL1 in a negative feedback loop. During the day, light degrades PER and CRY, allowing transcription to resume, thus establishing rhythmic transcription. In order to learn if body cells outside the SCN exhibit circadian clocks, Yoo et. al used a Luciferase bioluminescent reporter tied to the mPER2 gene promoter of the circadian rhythm; they found that mouse cells from the cornea, liver, lung, pituitary gland, retrochiasmatic area, and (dissected) tail continued to display bioluminescent light rhythms.
Scientists hypothesize that the SCN uses glucocorticoids as messengers to regulate subordinate clocks throughout the body. In a study dealing with the effects of glucocorticoids on lung tissue, researchers used the PER2:LUC reporter gene to find that Murine Clara cells exhibited sensitivity to glucocorticoids. Clara cells are tall, columnar, nonciliated cells that line respiratory bronchioles. They express glucocorticoid receptors as well as circadian rhythm genes.³ Furthermore, removing these cells from the tissue sample caused other lung cells to lose rhythmicity.³ In direct evidence supporting the hypothesis that glucocorticoids may influence circadian rhythms, rat fibroblasts have expressed clock genes in response to dexamethasone, a glucocorticoid. Liver, kidney, and heart cells all showed fluctuations in their rhythms in response to dexamethasone, while the SCN did not. It would make no sense for the SCN to accidentally self-regulate while attempting to regulate peripheral clocks, and this is confirmed above, as glucocorticoids act only on peripheral clocks without affecting the SCN.
A LINK BETWEEN THE CIRCADIAN RHYTHM AND IMMUNITY
The immune system is another domain governed by the circadian rhythm. In a study investigating the effects of BCG vaccine administration at different times of day, researchers found that volunteers vaccinated in the morning displayed stronger trained and adaptive immunity. Those injected in the morning showed higher levels of cytokines–cell secretions that broadly regulate immunity–in response to an administration of Staphylococcus aureus and Mycobacterium tuberculosis.
GLUCOCORTICOIDS IN AUTOIMMUNE DISEASE TREATMENT, AND THE CIRCADIAN CLOCK TIE-IN
Autoimmune diseases occur when the immune system attacks self-molecules following a breakdown of immunologic tolerance to autoreactive immune cells. Although there is no cure, patients are frequently prescribed synthetic glucocorticoids such as prednisone to make up for a lack of endogenous cortisol. An example is Rheumatoid Arthritis (RA), which, simply put, is joint inflammation.
The Hypothalamus Pituitary Axis (HPA) is an endocrine linkage that, among many functions, detects and combats inflammation. The HPA axis is governed by the SCN as well as positive and negative feedback loops. Levels of interleukin-6 (IL-6), a proinflammatory cytokine, rise just before dawn and fall as the day progresses. Cortisol is the body’s natural anti-inflammatory agent, and it is downregulated when IL-6 is on the rise, allowing for inflammation to take place. The HPA axis reacts to the increased inflammation by increasing cortisol levels, which alleviates inflammation.
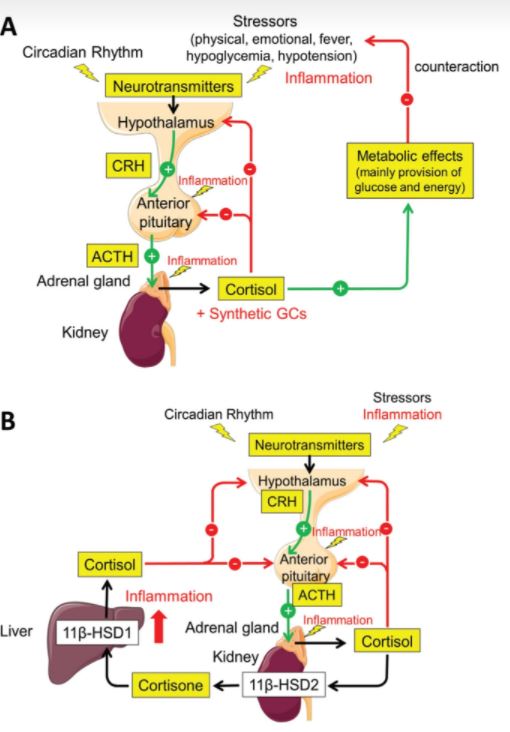
Note that cortisol is a negative regulator of the feedback loop, thereby shutting off its own production.
In order to study the action of the HPA axis, researchers simulated inflammation by injecting IL-6 into eighteen healthy males. As expected, the HPA axis released cortisol to combat the inflammation. But this response began to diminish with repeated injections of IL-6. This finding is consistent with the fact that RA patients suffer from chronically dampened levels of cortisol release by the HPA axis. The severe, prolonged inflammation caused by the disease dampens glucocorticoid release by the HPA axis. In fact, administering a glucocorticoid like prednisone with the goal of alleviating pain may backfire. Prednisone administered at 7AM was less effective, and we may speculate that it actually fed the negative feedback loop, prematurely terminating cortisol release (Figure 2).¹â° This results in increased inflammation.
To combat this, research has shown that a single morning dose of GC does not interfere with the natural rise and fall of cortisol.â¹ In practice, slow-release prednisone at 2AM was able to effectively suppress IL-6 release, while also leaving cortisol levels unaffected.¹â° Thus, timing prednisone administration is critical so as not to interfere with the body’s natural cortisol rhythm, which is, in part, circadian-controlled. Thus, the circadian timing of prednisone administration cannot be overlooked.
FUTURE DIRECTIONS
It would be wise for physicians to take into account the timing of glucocorticoid release before they administer prednisone. Slow release prednisone pills like Rayos are helpful in this regard. For example, taking Rayos at 10 pm results in a 2 am release. This ensures that the natural circadian cortisol rhythm is unperturbed. It is also vital for future studies to further elucidate the time-dependent dosage of glucocorticoids as these drugs cause harmful side effects down the line for many patients due to the instabilities they create.
REFERENCES
- Vitaterna, M. H., Takahashi, J. S., & Turek, F. W. (2001). Overview of circadian rhythms. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism, 25(2), 85—93.
- Yoo, S. H., Yamazaki, S., Lowrey, P. L., Shimomura, K., Ko et.Al, (2004). PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proceedings of the National Academy of Sciences of the United States of America, 101(15), 5339—5346.
- Gibbs, J. E., Beesley, S., Plumb, J., Singh, D., Farrow, S., Ray, D. W., & Loudon, A. S. (2009). Circadian timing in the lung; a specific role for bronchiolar epithelial cells. Endocrinology, 150(1), 268—276.
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C et.Al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000 Sep 29;289(5488):2344-7.
- de Bree LCJ, Mourits VP, Koeken VA, Moorlag SJ, Janssen R et. Al. Circadian rhythm influences induction of trained immunity by BCG vaccination. J Clin Invest. 2020 Oct 1;130(10):5603-5617.
- Smith, D.A. Germolec, D.R. Introduction to immunology and autoimmunity. Environ. Health Perspex. 107(Suppl 5). 1999.
- Son GH, Chung S, Kim K. The adrenal peripheral clock: glucocorticoid and the circadian timing system. Front Neuroendocrinol. 2011 Oct;32(4):451-65.
- Spies, C. M., Straub, R. H., Cutolo, M., & Buttgereit, F. (2014). Circadian rhythms in rheumatology–a glucocorticoid perspective. Arthritis research & therapy, 16 Suppl2 (Suppl 2), S3.
- Crofford LJ, Kalogeras KT, Mastorakos G, Magiakou MA, Wells J et.Al. Circadian relationships between interleukin (IL)-6 and hypothalamic-pituitary-adrenal axis hormones: failure of IL-6 to cause sustained hypercortisolism in patients with early untreated rheumatoid arthritis. J Clin Endocrinol Metab. 1997;
- Clarke L, Jessop DS, Hunt LP, Straub RH, Perry MG, Kirwan J. Alleviation of morning joint stiffness by low-dose prednisone in rheumatoid arthritis is associated with circadian changes in IL-6 and cortisol. Int J Clin Rheumatol. 2011
WRITTEN BY CHRIS MATTHEW CYRIL
Sixth College, B.S. Neurobiology, UC San Diego 2021
FROM SALTMAN QUARTERLY VOL. 18
To read the original version, please click here. To read the full version on our website, please click here. To read more individual articles, please click here.