INTRODUCTION
Tuberculosis (TB), a disease which primarily targets the lungs, is caused by the human pathogen Mycobacterium tuberculosis (Mtb). TB spreads when an infected person coughs, sneezes, or speaks, passing the bacteria onto someone else. Mtb is deposited into the lungs of the newly infected individual (resulting in pulmonary TB) and begins to proliferate, with a possibility of moving to other parts of the body such as the spine, brain, or kidneys.¹ Pulmonary TB symptoms include a bad cough, chest pain, and coughing up blood or phlegm, while symptoms of TB infection in other parts of the body vary depending on the organ in question.²
About 10 million individuals per year are still infected with TB, resulting in 1.5 million deaths worldwide.
DRUG RESISTANCE
As drug resistance becomes increasingly prevalent, researchers have the responsibility of developing novel drugs to fight multidrug resistant (MDR) and extensively drug resistant (XDR) TB. With the emergence of mycobacterial resistance, more than 500,000 individuals throughout the world become infected with resistant TB every year. These types of infections are very complicated and expensive to treat, especially in developing countries and underserved areas. Therefore, there is a high demand for novel and affordable antimycobacterial drugs. In order to develop new anti-tubercular drugs, it is essential to recognize the important role mycolic acids play in the virulence of Mtb. Mycolic acids, integral structural components of the Mtb cell wall, are long-chain fatty acids that are produced by fatty acid biosynthesis (FAB). Mtb employs both Type I and II fatty acid synthase pathways (FAS I and FAS II), producing various mycolic acids for a rigid and waxy cell wall.
FAS I AND FAS II MYCOLIC ACID PRODUCTION
FAS I is one giant multifunctional system of various protein domains, also referred to as a mega-enzyme, whereas FAS II is a system made up of multiple separate protein domains. Mtb employs the FAS pathways to form mycolic acids by moving an acyl substrate, which is attached to the acyl carrier protein (acpM), from one domain to the next. Each enzyme, also known as a partner protein, forms catalytically relevant interactions with the acpM and catalyzes its respective reaction. There are four iterative reactions involved in the FAS pathways: a condensation step, a reduction of a beta ketone, a dehydration step, and a final reduction of the enoyl intermediate into the final product.
PROTEIN-PROTEIN INTERACTIONS
According to a study by Veyron-Churlet, et al., the protein-protein interactions in the FAS II system of Mtb are extremely important for future drug development. Since these interactions are of great importance for the biosynthesis of the mycolate layer of the mycobacterium, they are also essential for the survival of the mycobacterium. These findings point to potential sites of inhibition in the FAS II pathway, which can possibly reduce the growth of Mtb.³
However, due to the very transient nature of the interactions between the acpM and its partner proteins, studying these interactions has not yet been successful.
In order to deal with the transient nature of some protein-protein interactions, the Burkart lab at University of California, San Diego has developed various biochemical tools that can be used to physically link two proteins together while they are interacting with each other. This covalently attaches the proteins to one another, completely eliminating the difficulty of evaluating transient interactions. These tools had previously been used for studying the E.coli system. Now, the Burkart lab strives to implement these techniques into the Mtb FAS II system, and study the protein-protein interactions of acpM and some of its partner proteins.
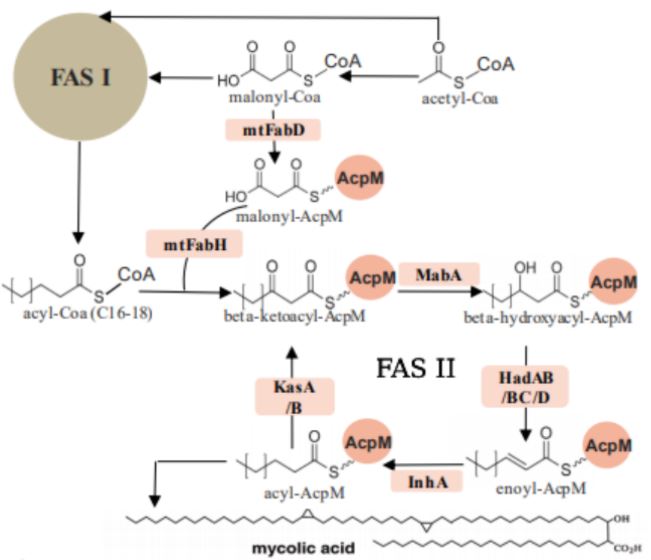
The FAS I and FAS II metabolic pathways of Mycobacterium tuberculosis show the formation of crucial structural components, mycolic acids.
The ketosynthase enzymes of Mtb FAS II, KasA and KasB, are two of the targets in this ongoing project. These enzymes perform the condensation step of the pathway to form a new carbon-carbon bond. Although both KasA and KasB catalyze the same reaction, there is a slight difference between the two enzymes. KasA, the essential elongation enzyme, catalyzes the initial elongations of the acyl molecule, and KasB, the nonessential elongation enzyme, is involved in the full elongation of the molecule (two carbons at a time until completion).
Other targets of interest of this current project are the recently identified dehydratase enzymes of Mtb FAS II, HadAB and HadBC, which perform the dehydration reaction to produce the trans-enoyl chain in the pathway.
As a tightly bound heterodimer, HadAB consists of two monomers, HadA and HadB, each with its specific function; HadB contains a critical catalytic dyad consisting of Histidine and Aspartate, whereas HadA has the ability to bind the fatty acyl substrate in its channel for catalysis to take place. HadAB is involved in the initial elongation of short mycolic acids, and HadBC, the other dehydratase of Mtb, catalyzes the elongation of mycolic acids to completion. The dehydration step is also a very important part of mycolic acid production, and therefore, an integral part of Mtb viability and survival. According to Belardinelli and Morbidoni, drugs currently used in the clinical treatment of TB, isoxyl and thiacetazone, affect the dehydration step of FAS II, catalyzed by HadAB and HadBC. However, the Had heterodimers are still a target for novel anti-tubercular drug development due to the rising drug resistance in Mtb.,â·
CONCLUSION
Mtb continues to be a worldwide health crisis. In particular, many developing countries are still facing the harshest effects of Mtb resistance against the current treatments. To fight this crisis, ongoing research strives to elucidate the protein-protein interactions involved in the fatty acid biosynthesis in Mtb. The potential findings of this research will aid in developing novel treatments and drug discovery.
REFERENCES
- How TB Spreads. (2016, March 11). Retrieved August 02, 2020, from https://www.cdc.gov/tb/topic/basics/howtbspreads.htm
- Signs & Symptoms. (2016, March 17). Retrieved August 02, 2020, from https://www.cdc.gov/tb/topic/basics/signsandsymptoms.htm
- Veyron-Churlet, R., Guerrini, O., Mourey, L., Daffé, M., & Zerbib, D. (2004). Protein-protein interactions within the Fatty Acid Synthase-II system of Mycobacterium tuberculosis are essential for mycobacterial viability. Molecular Microbiology, 54(5), 1161-1172. doi:10.1111/j.1365-2958.2004.04334.x
- Konno, S., La Clair, J., & Burkart, M. (2018, November 27). Trapping the complex molecular machinery OF Polyketide and fatty acid Synthases with Tunable Silylcyanohydrin crosslinkers. Retrieved April 22, 2021, from https://onlinelibrary.wiley.com/doi/full/10.1002/anie.201806865
- Cantaloube, S., Veyron-Churlet, R., Haddache, N., Daffé, M., & Zerbib, D. (2011). The Mycobacterium Tuberculosis FAS-II Dehydratases and Methyltransferases Define the Specificity of the Mycolic Acid Elongation Complexes. PLoS ONE, 6(12). doi:10.1371/journal.pone.0029564
- Belardinelli, J. M., & Morbidoni, H. R. (2012). Mutations in the essential FAS II β-hydroxyacyl ACP dehydratase complex confer resistance to thiacetazone inMycobacterium tuberculosis and Mycobacterium kansas ii. Molecular Microbiology, 86(3), 568-579. doi:10.1111/mmi.12005
- Dong, Y., Qiu, X., Shaw, N., Xu, Y., Sun, Y., Li, X., . . . Rao, Z. (2015). Molecular basis for the inhibition of β-hydroxyacyl-ACP dehydratase HadAB complex from Mycobacterium tuberculosis by flavonoid inhibitors. Protein & Cell, 6(7), 504-517. doi:10.1007/s13238-015-0181-1
WRITTEN BY LILIT VARDANYAN
Thurgood Marshall College, B.S. Human Biology, UC San Diego 2021, Burkart Lab, Department of Chemistry and Biochemistry
FROM SALTMAN QUARTERLY VOL. 18
To read the original version, please click here. To read the full version on our website, please click here. To read more individual articles, please click here.