Imagine possessing the superpower to resist blinking upon exposure to a flash of light. In real life, you would not be able to resist this urge due to the corneal reflex– a sudden and involuntary behavioral response. However, the inability to control this reflex does not make us any less valuable in society. Likewise, the same applies to individuals with Tourette Syndrome (TS), a neurodevelopmental disorder characterized by “tics,” or involuntary muscle contractions and twitches, vocalizations, and repetitive, stereotypical behaviors, such as tongue clicking and shoulder shrugging.
An official diagnosis of TS is independent of conventional laboratory or imaging tests. Rather, it is based on the patient’s history of symptoms, alongside a brief clinical examination to confirm that the tics are not associated with other causal factors, such as anxiety, stress, or even trauma. Although the severity differs from person to person, most affected individuals have a combination of simple and complex motor and phonic tics. Simple tics involve one muscle group and are brief, meaningless movements, such as eye blinking, neck jerking, and sniffing. On the other hand, complex tics, such as clapping, making obscene gestures, and tapping random objects repeatedly, often follow specific coordinated patterns between multiple muscle groups.
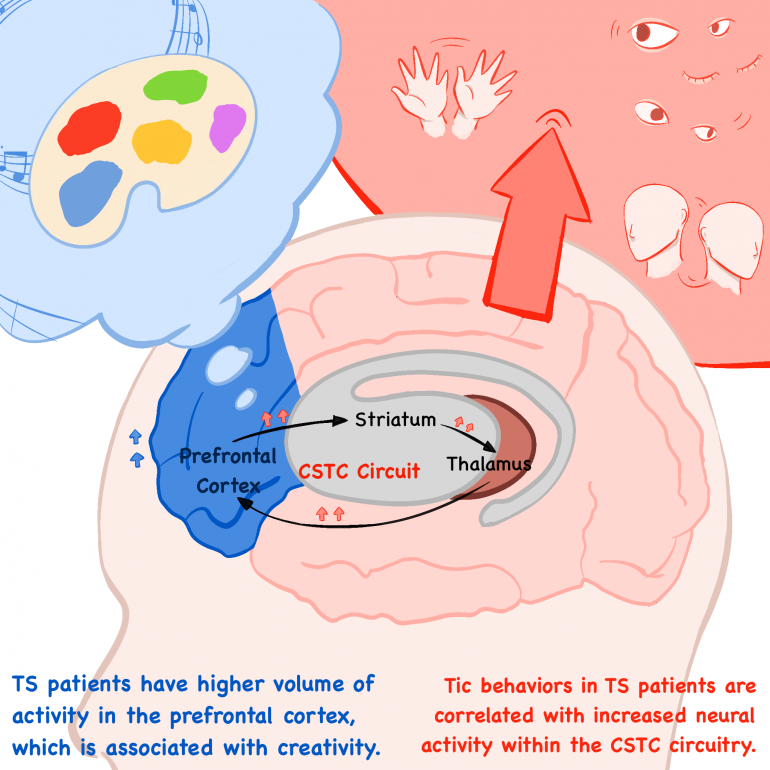
In most cases, the fluctuation of severity in motor and vocal tics decreases with age, given that a majority of individuals are diagnosed very early at around 5-7 years. However, a significant portion of TS adults live the entirety of their lives with mild tics on a daily basis. Often mistaken for a psychiatric condition, TS is actually a neurological movement disorder associated with an increased risk for comorbidities such as Attention-deficit/hyperactivity disorder (ADHD), Obsessive Compulsive Disorder (OCD), learning difficulties, and anxiety disorders. While behavioral therapy and pharmaceuticals are available treatments, the CDC suggests that there is no specified or proven cure for tics. From a neurobiological perspective, the exact site of genetic abnormality causing the tics still remains a heated topic within the scientific community. While it is largely assumed that localized disruptions to regions of the central nervous system have a role in TS, studies have shown that disturbances specifically to the large-scaled cortico-striato-thalamo-cortical (CSTC) circuits involved in controlling movements are a major hallmark of TS.
In a study conducted by Zhishun Wang and Tiago V. Maia, tic behaviors in TS patients were found to be attributed to increased neuronal activity within the CSTC circuitry involved in normal, voluntary motor behaviors in non-TS, or healthy, individuals. 13 TS patients and 21 healthy individuals were scanned using functional MRI, a technique used to measure high or low levels of brain activity on the basis of cerebral blood flow into specific regions, during the occurrence of both spontaneous and stimulated tics. The stimulated, or nonspontaneous, tics were induced by strong, brief magnetic impulses to the scalp that alter neural activity within the CSTC circuitry. Not only did the results indicate that there was higher neural activity within the sensorimotor regions of the CSTC circuits responsible but also that greater severity, or frequency, of tics was correlated with higher levels of activity in these regions. On the other hand, neural activity within the anterior cingulate and caudate–regions associated with the CSTC circuitry which control sensorimotor regions of the striatum–was significantly lower. Reduced activity of these regions would explain the inability of the brain to suppress the tic behaviors or the triggers within the sensorimotor regions that drive the tics associated with TS. Most significantly, these findings reiterate that tic behaviors in TS patients are attributed to both increased activation of the CSTC circuitry and reduced activity within the brain regions responsible for cognitive control of these motor networks.
There is a growing interest in understanding the pathophysiology and neurological basis of TS, and despite the many studies, little is known about the neurological condition. The informational scope of clinical trials and research is limited due to a variety of factors–small sample sizes, inconsistencies in age and medical background, a large range in severity of tics within clinical participants, and inability to control comorbidities’ effects on tics. Regardless, contemporary research increasingly points to the significance of alterations to the CSTC circuits in inducing TS-associated symptoms early in childhood.
On a more positive note, neurodevelopmental disorders are not only paired with cognitive impairments but are also frequently tied to certain enhanced functions or abilities. Studies have shown evidence indicating that TS patients have a higher volume of activity in the prefrontal cortex and possess alterations in connectivity between the prefrontal cortex and frontal cortex, which are both associated with creativity. Neurologically speaking, TS individuals also have higher dopaminergic activity, or stimulation of pathways dependent on the neurotransmitter dopamine, leading to increased creative thinking and behaviors. This creative potential in TS individuals is yet another reminder that neurodiversity is about normalizing and embracing differences.