Imagine: you’re at your local supermarket combing through the food storage containers aisle. You’re here on a mission to find the perfect tupperware. All those leftovers at home will go to waste if you don’t invest in some tupperware to properly store them. In other words, your potential breakfast and lunch are at stake, and that’s a price you’re not willing to pay. Now, the challenge lies in deciding which set of containers to buy. This one is the cheapest, but it’s too flimsy. There’s a matching set, but it’s not visually pleasing. The glass ones look nice, but they’re a bit too bulky and heavy. While contemplating your options, you can’t help but notice the fine print on one of the labels.
BPA-free.
Why does such a label exist? Does the presence of a label stating that a container is “BPA-free” mean BPA is bad for the body? Should we throw out everything that does not have the label?
Before going on a rampage to purge our homes of BPA, it is important to get familiarized with the intense discussion surrounding this little compound and how it is challenging traditional methods of toxicity testing.
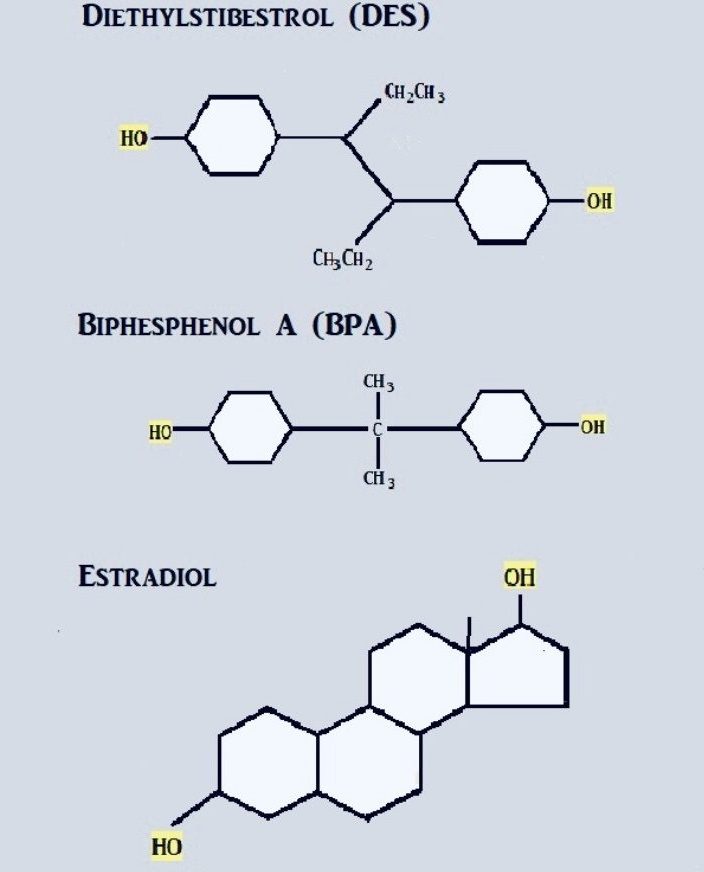
To begin, BPA stands for bisphenol A. This chemical compound was first synthesized in 1891, but it was not until the 1930s that BPA was discovered to have estrogen-like properties. BPA’s estrogenicity comes from its pair of hydroxyl “tips” that are located at each end of the molecule. This molecular arrangement is similar to that of estradiol, a common form of estrogen, whose hydroxyl “tips” are the main sites of biological activity. Because of this discovery, BPA became a candidate for an estrogen drug to treat issues related to menstruation and pregnancy, but that position was soon taken by a stronger synthetic estrogen called diethylstilbestrol (DES).
BPA would remain in the shadows until the 1950s when chemists discovered industrial uses for the compound that would eventually lead to its commercialization. It was found that BPA can be used to create two substances that are widely used today: epoxy resins and polycarbonates. Epoxy resin is a strong protective coating that is commonly used to coat floorings, seal teeth, and line the interior of food cans and water pipelines. On the other hand, polycarbonate is a strong, clear plastic that has many uses, one of which is in the production of storage containers for food and beverages. The versatility of BPA led the compound to become widespread, so much so that BPA could be found practically everywhere: reusable water bottles, food packaging, CDs, medical equipment, eyeglass lenses, and even some grocery receipts. The debut of BPA into the market brought convenience to society, and life in plastic seemed pretty fantastic.
The ubiquity of BPA meant that humans were often exposed to the compound, and this raised concerns about whether or not BPA posed any threat to human health. Due to early research showing that BPA had no notable effects on health, the U.S. Food and Drug Administration (FDA) declared that BPA was safe to use at low levels, and a tolerable daily intake of 50 μg/kg per day was established. Since then, however, a slew of research studies conducted by independent academic researchers began to find links between BPA and multiple health issues including infertility, cancer, heart disease, diabetes, and obesity at doses much lower than the FDA standard. These links were enough to categorize BPA as an endocrine disrupting chemical (EDC), which is quite unsettling considering how much of the human body operates on hormonal or endocrine activity. Nonetheless, this outcome is not surprising in light of BPA’s estrogen-like characteristics.
Despite all the incoming research advocating against BPA, the only change made in the FDA’s safety standards was a BPA ban in milk bottles, sippy cups, and baby formula in 2012, but this change was more of a formality than an active decision to protect public health. For several years, manufacturers producing baby products and toys voluntarily removed BPA from their products due to consumer concerns towards BPA. In 2011, the American Chemistry Council (ACC) petitioned the FDA to formally announce a partial ban in order to avoid confusion about whether these baby products sold in the U.S. contained BPA. The FDA complied, but stated that the ban did not correlate to a change in the FDA’s stance on BPA safety. For a federal agency that was established for the purpose of protecting public health by assessing the safety of various products, the late decision and adamant stance in the midst of many studies linking BPA to negative health effects was perplexing to say the least.
Even more perplexing is the discrepancy between the results of BPA research conducted by academic researchers and government scientists. Academic researchers claimed that BPA is a health hazard while the government scientists argued that there was no significant evidence suggesting a health threat. The continuous debate over the effects of BPA eventually led to a collaborative effort in 2012 called Consortium Linking Academic and Regulatory Insights on BPA Toxicity, or CLARITY-BPA for short. This collaboration between independent academic researchers and government agencies like the FDA, the National Institute of Environmental Health Sciences (NIEHS), and the National Toxicology Program (NTP) aimed to reconcile the discrepancy in results by using the same basic experimental framework to study the various effects of BPA. In 2018, the results of CLARITY-BPA were released to the public and ultimately concluded that BPA has low potential to cause health effects, even with continuous exposure.
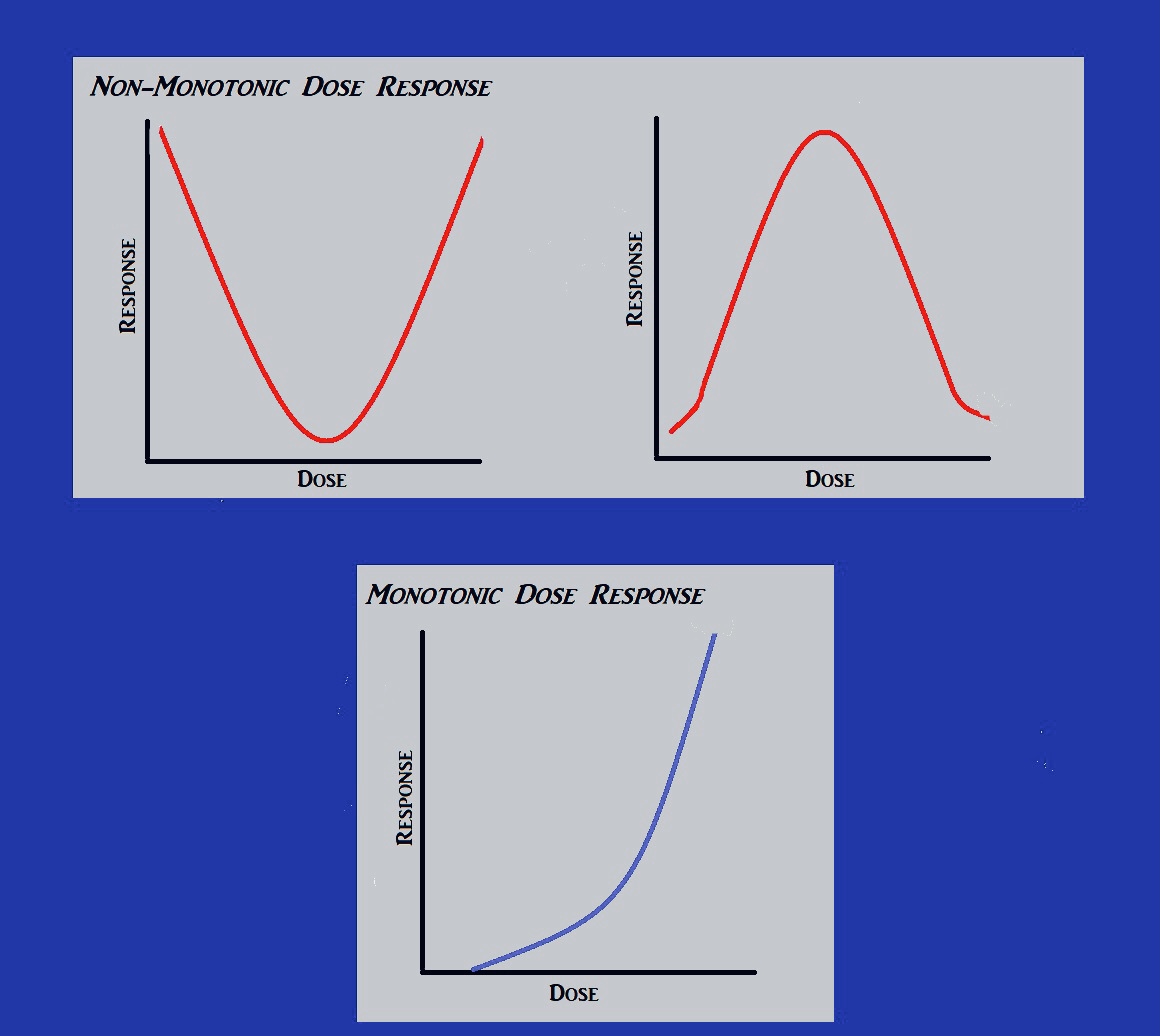
The study determined that no significant evidence indicated that BPA had a notable health effect, except in cases where higher doses were administered to the subjects. Academic researchers disputed this point, claiming that significant effects were indeed observed at lower doses but were dismissed as biologically irrelevant by those leading the study. Dr. Gail Prins from the department of pathology at the University of Illinois at Chicago shared that the scientists behind the government research studies “are toxicologists, they are not endocrinologists, and they don’t understand that effects at a low dose but no effects at high dose are very biologically plausible.” Prins’ statement challenges the research’s presumption that BPA operates under a monotonic dose-response relationship, where the effect of a substance increases with the dose administered. Instead, hormones and EDCs like BPA have been seen to operate under a non-monotonic dose-response (NMDR) relationship, where the effects are strongest in doses within a certain range. An NMDR relationship would explain why effects were observed at low doses of BPA but not at higher doses. It is for this reason that EDCs like BPA do not conform to traditional notions of toxicity and are causing toxicologists to revisit the old toxicological proverb, “the dose makes the poison.”
Apart from this point, the replicability of the study also came under criticism and called the reliability of earlier experiments into question. In order for research results to be worthy of scientific credibility, others must be able to replicate the study or conduct the same experiment and achieve similar results. However, replicability was not seen in the CLARITY-BPA study and prior studies by government agencies. Ethinyl estradiol, an active estrogenic ingredient in oral contraceptives, was used as a positive control to compare with and evaluate BPA’s estrogenicity in the study, but the data collected on the effects of ethinyl estradiol were not consistent with those found in previous experiments conducted by the FDA and NTP. This data inconsistency can be attributed to the different methods used to administer ethinyl estradiol. The earlier experiments introduced ethinyl estradiol to the animal subjects through food, whereas the substance was forcefully fed to the animal subjects with a gavage in the CLARITY-BPA study. Researchers speculate that the stress induced from forced feeding may have had a part in skewing the data. Additionally, the previous experiments kept the animal subjects in polycarbonate cages that have been shown to leach BPA. The background BPA exposure was either overlooked or not included in the evaluation, but when considering BPA’s estrogenicity, it likely played a role in influencing the resulting data for ethinyl estradiol. It is also important to note that although these earlier experiments were considered to be reliable, they were rarely conducted more than once, which indicates a lack of replicated studies to draw upon for comparison and credence. To put it simply, CLARITY-BPA has shed light on the flaws in current toxicology testing as well as the difficulty in monitoring background interferences.
Even though the study has ended, the results of CLARITY-BPA are still being heavily debated, but there are ways to reduce BPA exposure if one chooses to do so. BPA commonly enters the body through consumption of foods from BPA-containing products. Small amounts of BPA would seep into food and beverages from lined cans, plastic containers, and packaging, even more so when these BPA-containing products are placed under high heat. Thus, one way to reduce exposure is to avoid microwaving plastic food storage containers and packaging. Another way is to eat less canned foods, since the lining of cans contains BPA that can leach into the contents. Lastly, avoid plastics altogether (especially those with recycling codes 3 and 7 since they tend to contain BPA), and opt for glass, porcelain, and stainless steel options instead.
While looking for BPA-free alternatives, it makes sense to look for a “BPA-free” label, but such a label can be misleading. In order for these products to be free of BPA, there must be a replacement, and it is often the case that manufacturers simply substitute BPA with structurally and functionally similar compounds like BPS, BPF, BPAF and BPZ. The only difference between these compounds is a small chemical swap, similar to switching a blue lego block for a red one. As a result, these substitutes behave similarly and sometimes elicit a greater effect than BPA. Although many manufacturers have embraced the “BPA-free” label in their products, there are caveats that are unfortunately not always obvious.
While the CLARITY-BPA study is not perfect and did not resolve all the concerns surrounding BPA, many see it as a step towards improving current methods of assessing chemical safety. Despite this, the research behind BPA also highlights the difficulty in gauging how much of the results obtained in a strictly controlled laboratory setting are transferable to the actual human body, where there is a dynamic interplay with not only the body’s enzymes and hormones but also the various external chemicals that the body encounters in day-to-day life. CLARITY-BPA may have concluded, but the scientific investigation of BPA and its overall impact is far from over.
Who knew buying a new set of tupperware was so complicated?
Sources
- https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0120330
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2774166/
- https://www.medicalnewstoday.com/articles/221205#avoiding_exposure
- https://healthland.time.com/2012/07/17/fda-bans-bpa-from-baby-bottles-and-sippy-cups/
- https://www.nationalgeographic.com/science/2018/09/news-BPA-free-plastic-safety-chemicals-health/
- https://www.factsaboutbpa.org/safety-assessments/fda-research-bpa-safety/
- https://chemicalwatch.com/70439/academics-clash-with-fda-over-clarity-bpa-results
- https://www.the-scientist.com/news-opinion/a-landmark-study-on-bpa-leaves-scientists-at-odds-65004#:~:text=%E2%80%9CAlthough%20the%20CLARITY%20Core%20Study,their%20lives%2C%E2%80%9D%20he%20says
- https://www.researchgate.net/publication/331553037_Endocrine_disruptors_and_the_future_of_toxicology_testing_-_lessons_from_CLARITY-BPA
- https://www.webmd.com/children/what-is-bpa-is-it-safe#
- https://www.the-scientist.com/news-opinion/effects-of-bpa-substitutes-33729
All illustrations drawn by Svetlana McElwain
