By Ashley Medina | Online Reporter | SQ Online (2020-2021)
How much of you is you? Look down at your hands. Are you sure they’re yours? And what about the rest of your body– your eyes, skin, and internal organs? They seem to belong to you. For example, you can wiggle your fingers as you consider closing this article for fear of the existential crisis that reading it might provoke.
Don’t worry. Your body is yours. There may be a few microbiota living there, but your hands are on the wheel, right?
Thankfully, we can push the existential dread aside, and we don’t have to answer the philosophical question of what makes us fundamentally human. However, in order to prevent disease, our immune system does have to answer this question of what differentiates the self from the non-self.
The fundamental principle behind the success of the human immune system is the recognition of the self and its separation from the non-self. The distinction between the self and the non-self is mediated by tolerance mechanisms of the innate immune system in communication with the adaptive immune system. The innate immune system responds to a limited amount of patterns and structures of pathogens that it inherently recognizes. Specifically, the pattern recognition receptors (PRRs) recognize microbial-specific patterns such as bacterial cell wall constituents or double-stranded RNA. However, the PRRs cannot recognize and respond to the diversity of pathogens that is represented in the world. Instead, the adaptive immune system responds to a specific molecule of a pathogen, known as an antigen. In retaliation to an antigen, the adaptive immune system develops a counterattack using its T and B cells (Curry et al., 2017). The adaptive immune system is able to recognize specific pathogens because the PRRs that have detected pathogens can act as antigen-presenting cells and display an antigen to be recognized and targeted by T and B cells. In this way, PRRs are fundamental to the identification of pathogens and bridge the gap between the innate and adaptive immune systems (Lodish et al., 2016).
Whether or not the commensal microbiota is a part of “self” might be a philosophical question for us, but our immune system generally identifies them as such, and the microbes escape detection from the PRRs. And for a good reason: the microbiota plays a fundamental role in the function of the human body. For instance, gut microbiota are involved in maintaining the gut barrier through protection of intestinal epithelial cells; oral microbiota contribute to bone integrity and inflammation; and skin microbiota promote wound healing.
However, the functional role of microbiota homeostasis in the body has consequences. It has been observed that a disruption in the abundance of typical microbiota, dysfunctional microbiota, or a change in microbiota localization is correlated with immune-mediated disease. Immune-mediated diseases occur when tolerance mechanisms break down, causing harmless or beneficial cells to be targeted by the immune system.
Autoimmunity is a type of immune-mediated disease in which normal cells, or self-antigens, are recognized as pathogenic and targeted by T and B cells, leading to tissue damage. Microbial dysfunction has been implicated in a variety of different immune-mediated and specifically autoimmune diseases, particularly in relation to microbiota at the gut barrier. Microbiota have been found to be heavily involved in the maintenance of the gut barrier, meaning commensals work with the innate immune system in order to protect the intestine. For example, it has been shown that gut microbiota communication with mouse intestinal epithelial cells is necessary for the proper development of certain T cells. Additionally, disruption of the microbiota environment at the gut barrier has been associated with inflammatory bowel disease, a type of autoimmune disease that results in chronic inflammation of the gastrointestinal tract.
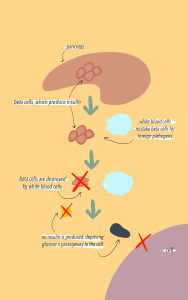
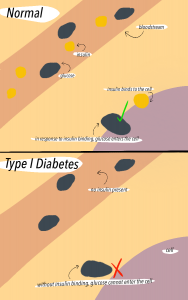
Another type of autoimmune disease associated with the gut barrier is Type 1 Diabetes. In Type 1 Diabetes, insulin-producing pancreatic beta cells are destroyed, which leads to complete loss of insulin production in most cases. In the pancreas, glucose and insulin regulate each other, meaning that loss of insulin leads to increased blood glucose levels. The lack of insulin prevents glucose from making it out of the bloodstream into the body cells. As a result, high blood glucose leads to complications such as kidney damage, cardiovascular disease, and nerve disease. The pathology of Type 1 Diabetes involves a complex interaction between environmental triggers, genetic factors, and the immune response, but it is also related to commensals.
Part of Type 1 diabetes pathology involves movement of commensals from the gut into the pancreas or pancreatic lymph nodes. There are multiple mechanisms in which microbiota can trigger the innate and adaptive immune systems to attack itself due to movement of microbiota. In one mechanism, gut microbiota translocation into pancreatic lymph nodes results in activation of NOD2 receptors, which are a type of PRR receptors that recognize a specific pattern of the translocated bacteria (Lodish et al., 2016). The activation of the NOD2 receptor then drives the differentiation of pathogenic Th1 and Th17 cells. Th1 and Th17 are helper T cells that release cytokines, which are cell signalling proteins of the adaptive immune system that, in this case, contribute to the destruction of pancreatic beta cells and subsequent loss of insulin production. When left untreated, increased glucose levels in the blood due to decreased insulin production exacerbates gut barrier dysfunction, which progresses microbial translocation in a vicious cycle.
Alright, being a little bit scared of the microbiota might be understandable. However, microbial dysregulation is only one part of the story. It is important to keep in mind that these gut microbiota are normal constituents of the body, and their dysfunction is not occurring spontaneously but rather as a result of a coupling between various environmental and genetic stressors. Along with various genes that have been associated with the development of Type 1 Diabetes, such as HLA (class II), environmental and lifestyle factors such as diet and even breast-feeding have been implicated as well.
The involvement of lifestyle in the progression of immune-mediated disease is not specific to Type 1 Diabetes. Other immune related diseases, such as allergies and celiac disease, have increased in incidence in the past several decades along with a dramatic alteration of commensal microbiota. Disrupted microbiota homeostasis has been associated with lifestyle changes such as increased use of processed food and antibiotics, indicating that such changes in modern life are contributing to unbalanced microbiota.
Due to the relationship between microbiota and lifestyle, appreciating the microbiota and studying their dysregulation in relation to the environment could potentially reveal preventative strategies to combat immune-mediated disease. Additionally, understanding the ways in which microbial homeostasis affects us can lead to new, exciting specialized care. For instance, identification of molecules in the microbiome that lead to a self-directed immune response is a promising avenue for fighting autoimmune disease. Microbiota-targeted antibiotics and vaccinations could also be beneficial.
So there’s no need to be worried about commensals. They might get a little confused sometimes, but they belong with you!
Even if they may have their tiny metaphorical fingers a little bit on the wheel…

Sources:
- https://www.nature.com/articles/s41579-020-0367-2#Sec4
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4925011/
- https://www.nature.com/articles/s41579-018-0014-3#:~:text=Growing%20evidence%20indicates%20that%20the,host%20behaviour%20for%20their%20benefit.
- Curry, L. A., Gain, M. L., Minorsky, P. V., & Reece, J. B. (2017). The Immune System. In Campbell Biology (11th ed., pp. 950—972). essay, Pearson.
- Lodish, H., Berk, A., Kaiser, C. A., Kreiger, M., Martin, K. C., Amon, A., … Bretscher, A. (2016). Immunology. In Molecular Cell Biology (8th ed., pp. 1080—1133). W.H.Freeman.
- https://wa.kaiserpermanente.org/healthAndWellness/index.jhtml?item=%2Fcommon%2FhealthAndWellness%2Fconditions%2Fdiabetes%2FinsulinProcess.html
- https://www.mayoclinic.org/diseases-conditions/hyperglycemia/symptoms-causes/syc-20373631#:~:text=High%20blood%20sugar%20(hyperglycemia)%20affects,taking%20enough%20glucose%2Dlowering%20medication.
- https://www.nature.com/articles/nrendo.2015.218
- https://www.cell.com/cell/fulltext/S0092-8674(04)00661-0?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0092867404006610%3Fshowall%3Dtrue
Illustrations by Natalie Madrigal
Photography by Jasmine Jung