Antibiotics are crucial in protecting humans from a large range of bacterial diseases. Scientists have created several types of antibiotics that are now prescribed regularly to treat many infections. Unfortunately, the misapplication of antibiotics for viral infections and the unnecessary use of antibiotics as household cleaners have increased antibiotic resistance, in which bacteria evolve over generations, such that these treatments are no longer effective. In addition to killing harmful bacteria, the overuse and misuse of antibiotics eliminates beneficial bacteria, creating ideal conditions for malignant populations to grow and infect the host. A commonly-cited example of an antibiotic-resistant bacteria strain is methicillin-resistant Staphylococcus aureus (MRSA). Staphylococcus aureus has grown resistant to the antibiotic methicillin because of horizontal gene transfer, which lets bacteria distribute their genetic information among various species, augmenting the number of resistant genes a strain has.¹,¹¹ Consequently, MRSA develops antibiotic resistance and has the potential to invade immune systems and kill individuals. Over time, bacterial resistance has increased faster than the discovery and distribution of new antibiotics.¹ This creates a pressing need for a new source of antibiotics that are effective against numerous bacteria strains. This review highlights a possible solution: equisetin. Found in the marine sponge species Fusarium equiseti, equisetin poses a breakthrough in the field of medicinal chemistry, with the ability to fight multi drug-resistant bacteria by inhibiting bacterial cell wall synthesis.
One key chemical mechanism driving the effectiveness of antibiotics is their ability to block bacterial cell wall synthesis. Beta-lactam antibiotics are a family of antibiotics containing a beta-lactam ring, known as beta-lactamase. This four-membered cyclic amide ring⁷ attaches to the beta-lactam receptors found in bacterial cell walls and inhibits its function and repair mechanisms, resulting in the cells breaking apart.⁸ Marine fungi species are known to carry these lactamases. Equisetin, derived from one such species, contains this ring, contributing to its efficacy in overcoming antibiotic resistance. These findings suggest that more fungal species should be observed for the possibility of discovering more antibiotic species with capabilities similar to equisetin.
Marine sponges are a well-known source of medicinal biosynthetic compounds.² They contain numerous microorganisms—cyanobacteria, algae, and viruses—which biosynthesize compounds that can be used as immunosuppressants, neurodegenerative disease supplements, and antibiotics. Many antibiotics in the current pharmaceutical market are derived from marine organisms. For example, cephalosporins, used to treat skin infections, are derived from the fungi genus Acremonium.² These marine organisms produce polypeptides that act as antibiotics by permeabilizing the cell membrane of bacteria, allowing toxic substances to enter and destroy bacterial particles.³ Another example is myticusin-1, derived from the mussel Mytilus coruscus, which has the potential to target bacterial species such as Bacillus subtilis, Staphylococcus aureus, Sarcina luteus, and Bacillus megaterium by blocking bacterial cell wall synthesis.¹³
This review primarily focuses on a research article in which scientists performed multiple experiments with equisetin to determine its efficacy. The first step for the scientists was identifying which fungal species contained equisetin. This was done using antiSMASH, a computer database containing software that recognizes secondary metabolites.⁴ Using antiSMASH, the scientists found a gene cluster in the marine fungi species F. equiseti that was 91-96% identical to the compound equisetin. After identification in the fungi species, this molecule was isolated from deep-sea sediment and fermented in Petri dishes.⁵ The equisetin was then purified and separated from the sponge using an analytical chemistry technique called high-performance liquid chromatography (HPLC). The product of this method was a pale red powder: equisetin in a functional form for experimentation.
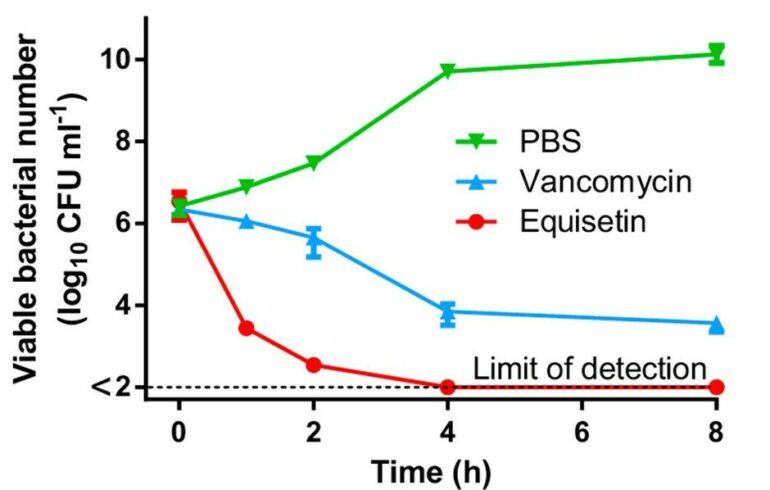
Once the equisetin was procured, multiple bioassays were performed to examine the antibiotic abilities of equisetin against multi drug-resistant (MDR) bacteria.⁵ Experiments were conducted with gram-positive bacteria (bacteria containing thick cell walls), such as MRSA. In these experiments, equisetin proved more effective against multiple strains of these bacteria than the antibiotic vancomycin. This was shown with equisetin’s lower minimum inhibitory concentration (MIC), defined as “the lowest concentration of an antimicrobial that will inhibit the visible growth of a microorganism after overnight incubation” (Figure 1).⁵
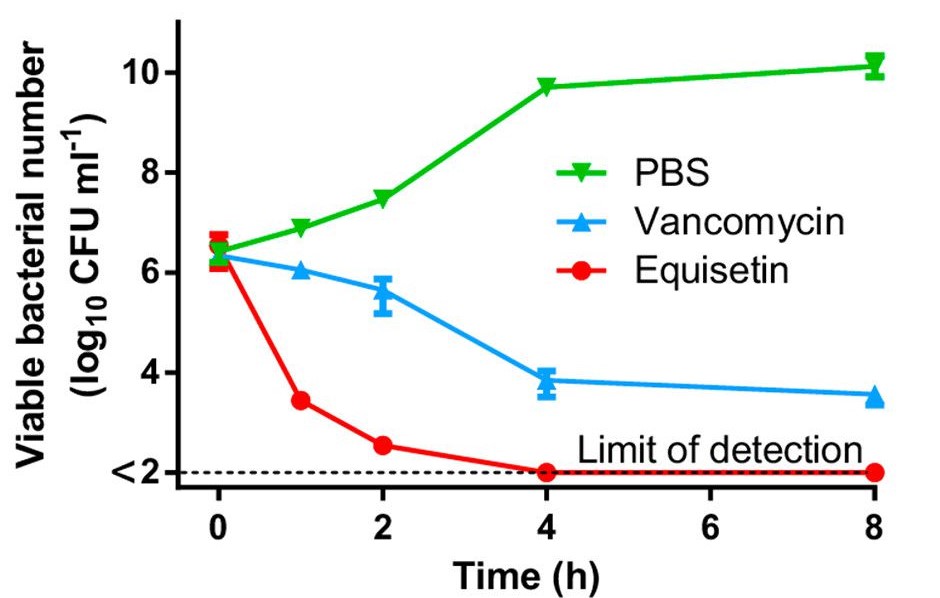
Equisetin on its own did not perform better than vancomycin on gram-negative bacteria, as both resulted in a MIC over 128 after being tested on strains such as Escherichia coli and Pseudomonas aeruginosa. However, further experimentation showed that equisetin acts well in synergy with certain antibiotics and helps eliminate such bacterial strains. A previous experiment measured the efficacy of seven gram-negative bacteria-targeting antibiotics. Among the seven, colistin had the best synergy with equisetin. When combined, the treatments performed very well against E. coli and S. aureus, requiring a lower dosage to achieve the efficacy of colistin alone.⁵ In fact, the equisetin-colistin combination is even powerful against a colistin-resistant strain of E. coli (Figure 2).
Using multiple strains of E. coli with different concentrations of antibiotics revealed that a combination of equisetin and colistin inhibited 100% of the bacterial strains and killed 99% of the bacteria within 1 hour.⁹ With colistin and equisetin established as a powerful synergistic duo, the scientists performed studies on the biological mechanisms behind their efficacy. Using fluorescent dyes, they discovered that the equisetin-colistin combination breaks down both the outer membrane and plasma membrane of the bacteria, making it more susceptible to outsider attacks.
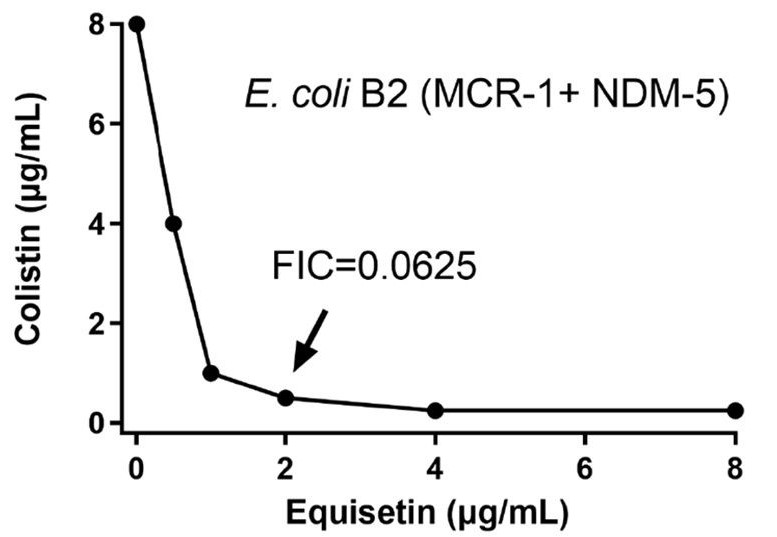
In an experiment where staph bacteria were treated with low levels of equisetin over 100 days to develop resistance, the MIC required to kill the bacteria increased only by a factor of four and the bacterial growth rate flattened.⁵ The MIC for oxacillin, the standard antibiotic used for staph infections, increased by a factor of 64. A higher MIC indicates that more of the antibiotic is required to eliminate the bacterial strain. Thus, compared to oxacillin, equisetin is a far more effective repressor of resistance (Figure 3).
Furthermore, the bacterial strains treated with equisetin were shown to develop collateral sensitivity; as the strain becomes resistant to one antibiotic, it is more susceptible to another antibiotic. Equisetin-treated bacterial strains became vulnerable to many different antibiotics such as daptomycin, gentamicin, and erythromycin, while developing resistance to equisetin. The MIC of equisetin required to eliminate antibiotic-resistant strains of certain bacteria such as S. aureus and VRE is two times less than what is required to eliminate the wild-type strain of the same bacteria. This suggests that a lower antibiotic dosage is required for bacteria strains treated with equisetin that are resistant to other antibiotics.
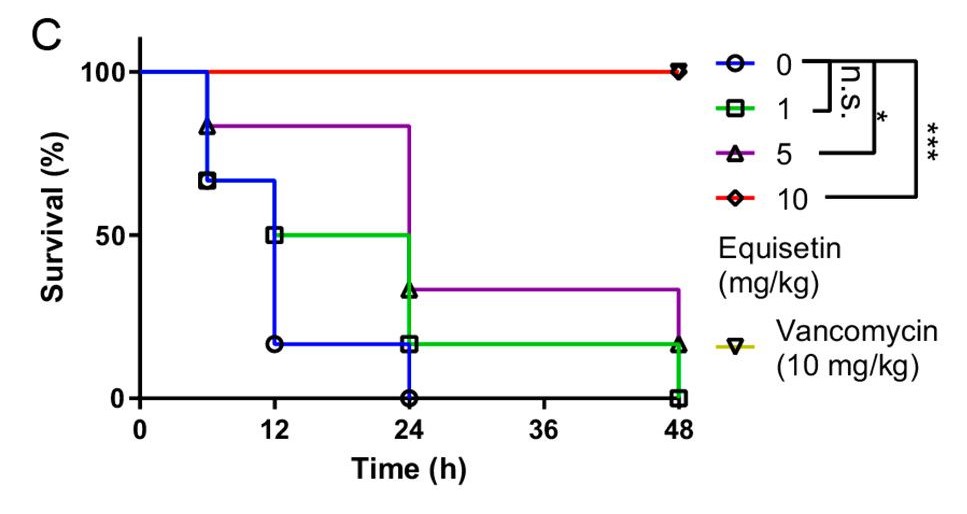
Finally, equisetin was injected into animal models to analyze its in-vivo efficacy. In the Galleria mellonella larvae model, MRSA was injected first, followed by equisetin at a dose gradient ranging from 1 mg/kg to 10 mg/kg. The results showed that a standard dose of 10 mg/kg of equisetin can inhibit MRSA from infecting the larvae host and, as a result, decrease bacterial load (the quantity of bacteria in an organism).⁵ In addition, the larvae models injected with equisetin had a higher survival rate than the ones injected with the antibiotic vancomycin. Similar results were produced in the rat models: the equisetin-injected mice experienced higher survival rates and these mice decreased bacterial loads in multiple organs (Figure 4). This suggests that equisetin has the potential to be a novel antibiotic for other animals and possibly even humans.
Through experiments with bacterial cell cultures and in-vivo models, scientists have shown that equisetin can be effective in killing many strains of gram-positive and -negative bacteria while serving as an aid to other antibiotics like colistin. Scientists’ next steps could be to extend previous mice experiments into primate studies, eventually culminating in human trials. This would be useful in determining whether equisetum is effective in humans, while also revealing certain risks and side-effects that were not seen in the bacterial and mice models. As more fungal species such as F. equiseti continue to be found, strides will be made towards alleviating the medical problem of antibiotic resistance, providing necessary treatment for many patients globally.
Acknowledgements
This review was completed as part of a project titled “Chemistry in Context” in Dr. Stacey Brydges’ General Chemistry Class. I would sincerely like to thank Dr. Brydges for her assistance and guidance throughout this project. In addition, I would also like to express my gratitude for the researchers of the original paper that this review is based on.
References
- Jensen, S. O.; Lyon, B. R. Genetics of Antimicrobial Resistance in Staphylococcus Aureus. Future Microbiol. 2009, 4 (5), 565–582.
- Häder, D.-P. Marine Sponges: Source of Novel Biotechnological Substances. In Natural Bioactive Compounds; Elsevier, 2021; pp 363–379.
- Polypeptide Antibiotics: Bacitracin, Colistin, Polymyxin, https://www.msdmanuals.com/professional/infectious diseases/bacteria-and-antibacterial-drugs/polypeptide-antibiotics-bacitracin,-colistin,- polymyxin-b (accessed May 18, 2021).
- antiSMASHbacterialversionhttps://antismash.secondarymetabolites.org/#!/about(accessed May 18, 2021).
- Chen, S.; Liu, D.; Zhang, Q.; Guo, P.; Ding, S.; Shen, J.; Zhu, K.; Lin, W. A Marine Antibiotic Kills Multidrug-Resistant Bacteria without Detectable High-Level Resistance. ACS Infect. Dis. 2021, 7 (4), 884–893.
- HPLC | High performance liquid chromatography https://www.youtube.com/watchv=ZN7euA1fS4Y (accessed May 31, 2021).
- Gao, M.; Glenn, A. E.; Blacutt, A. A.; Gold, S. E. Fungal Lactamases: Their Occurrence and Function. Front. Microbiol. 2017, 8, 1775.
- Kohanski, M. A.; Dwyer, D. J.; Collins, J. J. How Antibiotics Kill Bacteria: From Targets to Networks. Nat. Rev. Microbiol. 2010, 8 (6), 423–435.
- Zhang Q, Chen S, Liu X, Lin W, Zhu K. Equisetin Restores Colistin Sensitivity against Multi-Drug Resistant Gram-Negative Bacteria. Antibiotics (Basel). 2021;10(10):1263. Published 2021 Oct 18. doi:10.3390/antibiotics10101263
- Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001 Jul;48 Suppl 1:5-16. doi: 10.1093/jac/48. suppl_1.5. Erratum in: J Antimicrob Chemother 2002 Jun;49(6):1049. PMID: 11420333.
- Sun, D., Jeannot, K., Xiao, Y., & Knapp, C. W. (2019, August 27). Editorial: Horizontal gene transfer mediated bacterial antibiotic resistance. Frontiers. Retrieved March 6, 2022, from https://www.frontiersin.org/articles/10.3389/fmicb.2019.01933/full
Written by Akshay S. Bharadwaj
B.S., Microbiology, Class of 2024, Warren College