Over 1.3 billion people are afflicted with some form of vision impairment, which can mean these individuals require glasses or have a level of blindness. While the decline of vision with age is generally considered to be a naturally occurring and inevitable process, not all instances of vision impairment can be dismissed as products of aging. Instead, some afflictions result from various pathologies and conditions that develop in the eye. Retinal diseases are a class of ocular diseases that can damage the retina, the thin layer of tissue located at the back of the eyeball. Retinal diseases are relatively common and affect over 200,000 people in the United States alone. Retinal problems are very serious because if left untreated, they can lead to permanent blindness.¹
Before expanding on retinal diseases, it is important to characterize the retina and understand how it functions. In the retina, light signals are transformed into electrical signals. Various interconnected cell types, such as photoreceptor cells, bipolar cells, horizontal cells, and retinal ganglion cells, work together to accomplish this key process. The retina sends the information collected by these cells through the optic nerve to the brain, a process that enables sight. In the human retina, two types of photoreceptor cells, rods and cones, are responsible for dim light vision and daylight vision (including color), respectively. Rods are located mainly in the peripheral retina, and cones are concentrated in the macula, the small, central-most portion of the retina that provides high-resolution vision. Photoreceptor cells transmit visual signals to bipolar cells which then pass these messages on to retinal ganglion cells. Ultimately, all the signals are gathered in the optic nerve and transmitted to the brain.² Retinal degeneration is a type of progressive neurologic disorder that can be caused by genetic mutations, environmental or pathologic retinal damage, or a combination of both. This group of disorders is characterized by different types of retinal cell loss. Age-related macular degeneration and retinitis pigmentosa could involve a reduction in the retinal pigment epithelium or photoreceptor cells, while glaucoma involves retinal ganglion cell death.³
Currently, the process of retinal degeneration is both incurable and not completely understood. However, Karl Wahlin, Assistant Professor of Ophthalmology at UC San Diego, aims to study the root causes of retinal degenerative diseases by experimenting with stem cell-derived retina tissue. Dr. Wahlin’s research explores the directed differentiation of pluripotent stem cells (PSCs), naïve cells that have the ability to mature into any one of numerous cell types, and their application in examining retinal development and disease. One subsidiary but vital aim of Dr. Wahlin and his lab is to produce fully functional retinal structures from PSCs. Human pluripotent stem cells (hPSCs) possess two key intrinsic properties that distinguish them from all other cell types. First, they display the potential to differentiate into all somatic cell lineages and even developing embryonic tissue. In its earliest recognizable stage of growth, developing embryonic cells are capable of becoming tissue or even multi-layered organs. Secondly, hPSCs are able to maintain long telomeres, regions of nucleic acid that protectively tail DNA, allowing hPSCs to be replicatively immortal and making them a reliable, replenishable source of cells for differentiation and translational research. Thus, PSCs, characterized by their unlimited proliferation capacity and ability to give rise to any cell type in the body, are a promising source for cell replacement therapy. Researchers at the Wahlin lab evaluated the directed differentiation of hPSC-derived retinal cells. Through their efforts, the researchers found that hPSCs can differentiate into retinal pigment epithelium, retinal neurons, and photoreceptor cells. In other words, which can organize into self-forming, multi-layered retinal tissues, essentially giving rise to their own 3D-mini-retinas.
During embryogenesis, the eye field first appears as an optic vesicle originating from the diencephalon. The distal tip then inverts itself to form a double-layered optic cup, with the outer layer forming the retinal pigment epithelium and the inner layer becoming the neural retina. Rather than optic cups, the Wahlin lab uses stem cell-derived 3D-retinas that are single-layered sheets of the neuroepithelium, which are similar to optic vesicles. These lack both an optic stalk and an adjacent retinal pigment epithelium. As these vesicles mature, they are simply referred to as “retina cups” or “mini-retinas”. These 3D-mini-retinas offer exciting opportunities to study the detailed mechanisms of retinal degeneration and provide new models for drug discovery and cell-based therapeutics.
The Wahlin lab makes use of pre-existing procedures to develop conditions that support the growth of these mini-retinas. However, they have modified what is known as the forced aggregate protocol, a method for cultivating stem cell tissue, for generating hPSC-derived 3D-retinas. At Wahlin lab, the protocol was modified for growth medium composition, O2 concentration, and aggregate size. The method followed at the Wahlin lab lead to floating 3D-optic vesicle-like structures within twelve days, and in long-term cultures, leads to advanced photoreceptor development, including rod and cone outer segments and neurotransmitter expression.
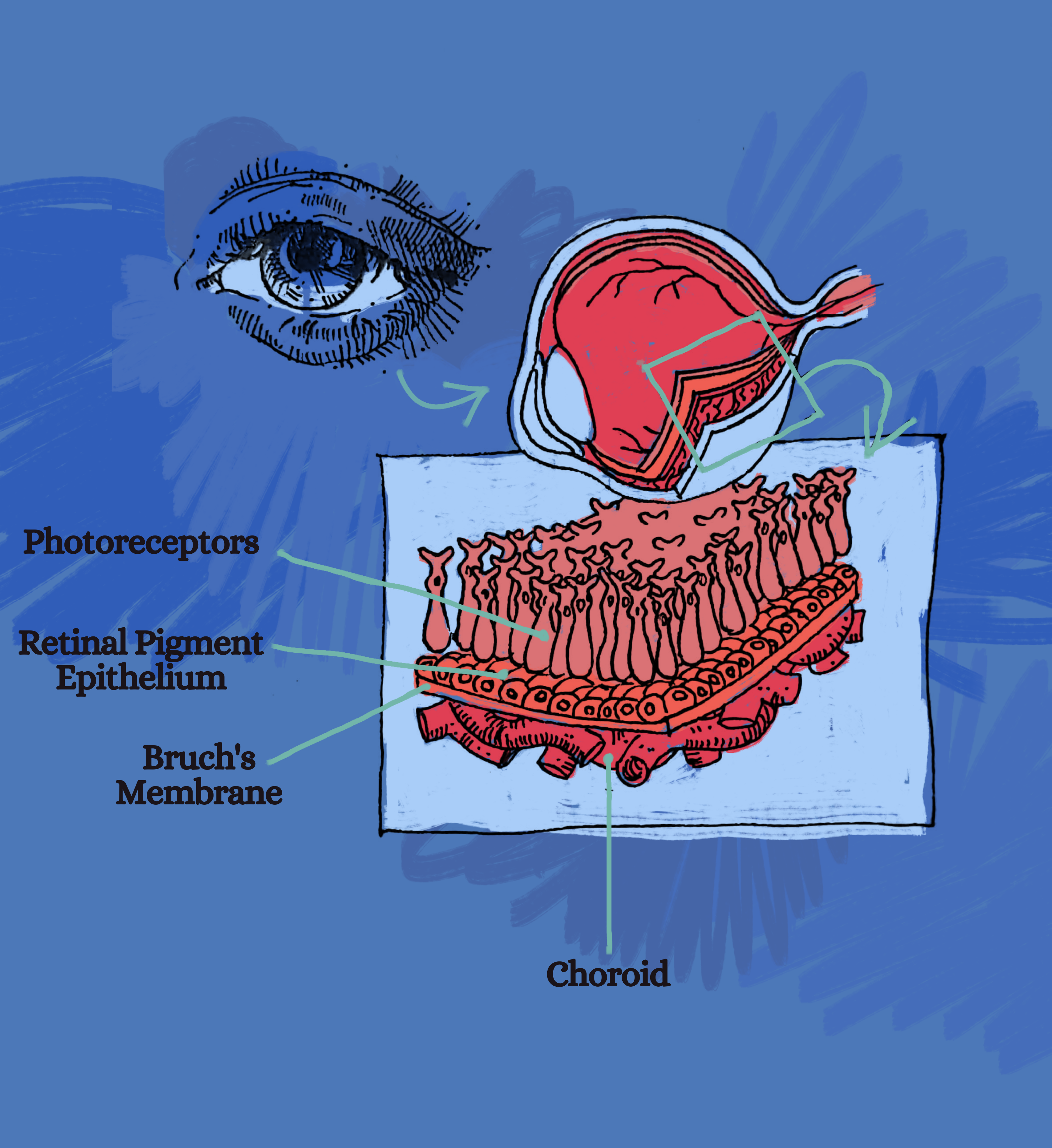
Four layers of the retina are depicted here: photoreceptors, which include both rod cells and cone cells, the retinal pigment epithelium, Bruch’s membrane, and the choroid.
Retinal cups are cultured in three different cell lines. The cell line is a general term that applies to a defined population of cells that can be maintained in culture for an extended period of time, retaining the stability of certain phenotypes and functions. Cell lines are usually clonal, meaning that the entire population originates from a single common ancestor cell. Because of the clonal nature of cell lines, researchers avoided cultivating cell-line-specific structures as such structures would create biases in the composition of the tissue. Thus, in order to ensure that the optic vesicle formation was not cell-line specific, researchers worked in parallel with three hPSC lines: the IMR90.4, EP1iPSC, and H7 ESC lines.
The generation of moderately sized optic vesicles takes seventeen days. The time taken to cultivate vesicles was the first step researchers worked to optimize. Based on the hypothesis that growth medium composition, O2 concentration, and aggregate size of optic vesicle could each impact cell differentiation, the researchers at the Wahlin Lab systematically optimized each of those parameters.
In regards to the expansion media, cells were cultured in a growth medium solution that was designed to support the expansion of a population of microorganisms or cells via proliferation. A Neural Induction Medium is a defined, serum-free medium for the neural induction of human embryonic stem cells and induced PSCs. This medium enables the highly efficient generation of neural progenitor cells, the precursor cells to retinal neurons. To optimize the medium composition, the lab found a serum-free formulation optimized to grow and maintain undifferentiated embryonic stem cells, which ended up being valuable in supporting vesicle formation.
Next, the researchers at Wahlin lab sought to optimize O2 concentration. They found that hypoxia, or low levels of oxygen, can improve the survival, pluripotency, and proliferation of hPSCs. Relative to aggregates initiated in 20% O2 (normal oxygen levels or normoxia), aggregates maintained in 5% O2 (hypoxia) conditions for an additional day demonstrated increased viability. By day eight, hypoxic aggregates had more vesicles per aggregate and were larger in size than their normoxic counterparts.
Finally, the researchers examined the concentrations at which stem cells could be cultured. The vesicles were ultimately maintained at low density since at high density they often coalesced into caterpillar-like chains with necrotic cores. Poor quality vesicles with an opaque appearance or with signs of necrosis were regularly discarded. Vesicles that were insufficiently excised were further trimmed to approximately 500 microns to prevent overgrowth in subsequent weeks.
After one month of periodic grooming in the form of removal of non-retinal cup-like structures and trimming of overgrown vesicles, the 3D-translucent retinal cups appeared relatively homogeneous, with minor differences in shape and size.
The next step in the process of producing fully functional retinal structures from pluripotent stem cells was to check for necessary elements of a retina in the retinal cups: an important feature in the retina is the synaptic ribbons. Release of the neurotransmitter glutamate at the ribbon synapse facilitates information transfer from photoreceptor cells to bipolar cells. Conventional neurons encode information by changes in the rate of action potentials, but for complex senses like vision, this is not sufficient. Ribbon synapses enable neurons to transmit light signals over a dynamic range of several orders of magnitude in intensity. Synaptic ribbons are found in mature rods and cones and are essential for retinal function. In the outer plexiform layer of the retina, ribbons form a tripartite junction with bipolar and horizontal cell dendrites. In order to ensure that the presence of these structures was preserved in the retinal cups, an immunohistochemistry (IHC) test was conducted for postsynaptic density-95 (PSD95) and C-terminal binding protein (CtBP2). IHC stainings use the principle of antibody-antigen binding specificity to detect target proteins in cells. The CtBP2 antibody recognizes a transcriptional repressor and a synaptic protein, RIBEYE. The presence of RIBEYE indicates the presence of ribbon synapses since RIBEYE interactions are required for the synaptic ribbon to bind to the retina.
Moreover, the synaptic function of photoreceptors in the eyecup was assessed using electrophysiological recordings to measure total membrane capacitance. Photoreceptors were clamped at -65mV and then depolarized to -10mV to trigger vesicle release. Stimulus-evoked spikes in capacitance unaccompanied by simultaneous changes in either the membrane or series resistances were taken as evidence of vesicle exocytosis.
The retinal cups were also tested for All-trans retinoic acid (ATRA). Although ATRA has well-documented boosts in retinal development, its prolonged presence can hamper maturation. Based on this, researchers treated retinal cups with 500nM ATRA every day from 20 days after retina formation until 120 days before photoreceptor maturation. Shortly thereafter, small sprouts began to emerge from the retinal cup surface, and after 160 days, photoreceptor outer segment-like structures were present. Variability in the length and onset of cup formation was observed. The segment growth was self-limiting, reaching a terminal length of approximately 39μm, a range similar to that reported in vivo. Unlike the cone-rich fovea in vivo, rods and cones in retinal cups were generally evenly dispersed across the mini-retinas. The retinal pigment epithelium also frequently grew on retinal cups opposite to the retina or as independent spheroids with honeycomb-shaped polygonal morphologies.
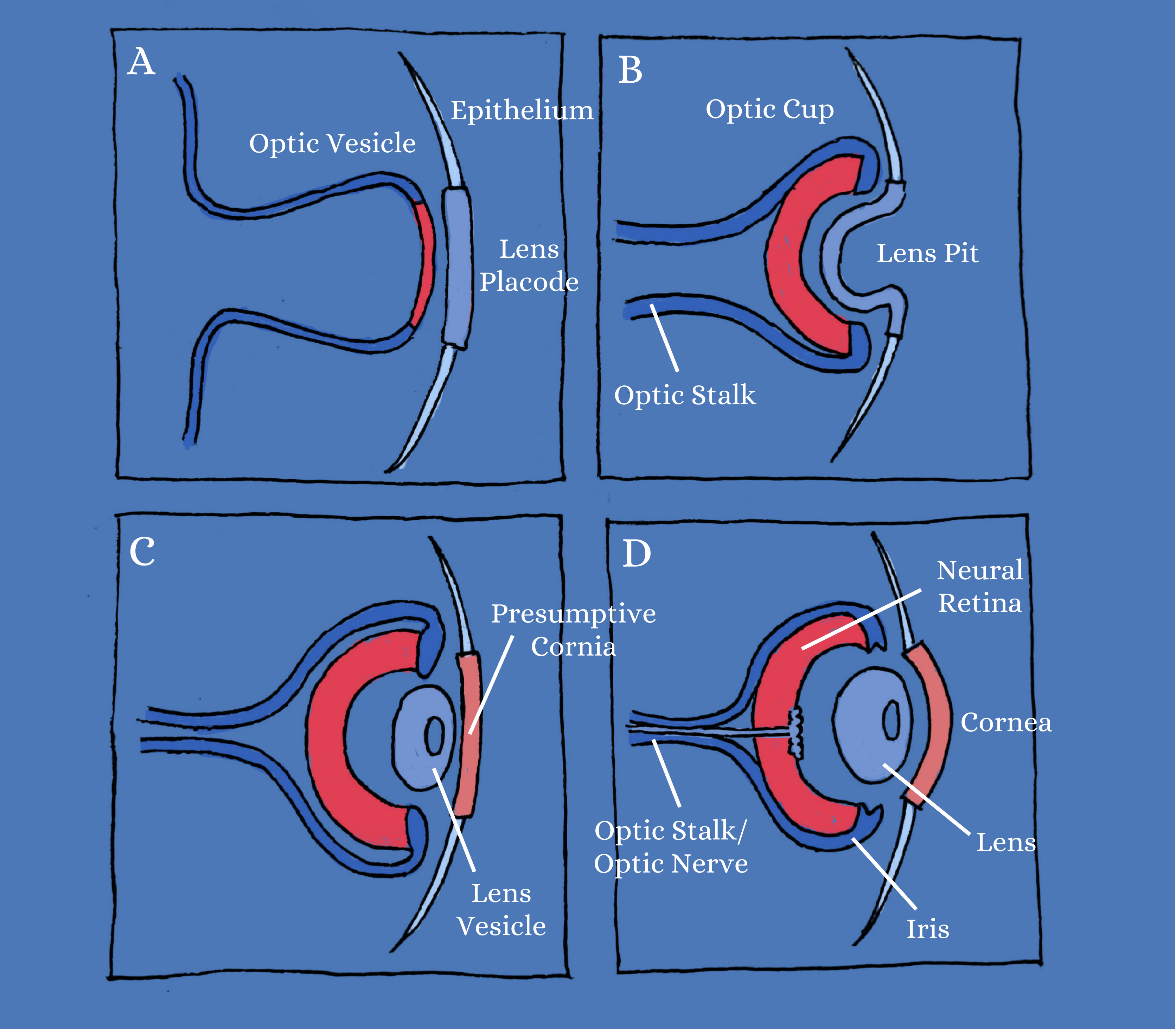
the Optic Vesicle
The optic vesicle forms the foundation for what eventually becomes the optic cup, which includes the optic stalk, the optic nerve, the neural retina, and iris. Epithelial cells differentiate to form the cornea and lens.
hPSCs have become invaluable tools to investigate the different stages of retinal degeneration and to help tailor therapeutic strategies of the future. Although the Wahlin Lab’s retinal cups are not prepared to serve as a cure for retinal degenerative diseases without thorough testing on animals and/or humans, the research conducted to develop the retinal cups is a significant stepping stone on our path towards a cure. While the retinal cups do not cater to all retinal degenerative diseases, they do provide a good model for researchers to study the causes of these diseases. In cases of advanced degeneration, hPSCs that have differentiated into retinal cells could be transplanted into the eye to replace lost cells or to support remaining photoreceptors through cytoplasmic exchanges. While significant roadblocks need to be addressed, the rapid development of more physiologically relevant cellular models that accurately capture the biological complexity of the retina in vitro, like the Wahlin Lab’s stem cell-derived retina structures, bring these expectations closer to reality.
REFERENCES
- Davidson, F., Steller, H. Blocking apoptosis prevents blindness in Drosophila retinal degeneration mutants. Nature 391, 587—591 (1998). https://doi.org/10.1038/35385
- Hoon, Mrinalini, et al. “Functional Architecture of the Retina: Development and Disease.” Progress in Retinal and Eye Research, Pergamon, 28 June 2014, www.sciencedirect.com/science/article/pii/S135094621400038X?casa_token=QQq1TD0bBocAAAAA%3ALT9Op5yxB39MsIW9MErBLsJ0bjsQ9xm59nOLwLB36lauakwBpqKA8pz_lMWOjPW3QmxQjIKnzg.
- Huang, Y., Enzmann, V. & Ildstad, S.T. Stem Cell-Based Therapeutic Applications in Retinal Degenerative Diseases. Stem Cell Rev and Rep 7, 434—445 (2011). https://doi.org/10.1007/s12015-010-9192-8
- Singh, R., Cuzzani, O., Binette, F. et al. Pluripotent Stem Cells for Retinal Tissue Engineering: Current Status and Future Prospects. Stem Cell Rev and Rep 14, 463—483 (2018). https://doi.org/10.1007/s12015-018-9802-4
- Wahlin, K.J., Maruotti, J.A., Sripathi, S.R. et al. Photoreceptor Outer Segment-like Structures in Long-Term 3D Retinas from Human Pluripotent Stem Cells. Sci Rep 7, 766 (2017). https://doi.org/10.1038/s41598-017-00774-9
WRITTEN BY VIDISHA MARWAHA
Vidisha is a Human Biology Major from Earl Warren College. She will be graduating in 2024.
FROM SALTMAN QUARTERLY VOL. 18
To read the original version, please click here. To read the full version on our website, please click here. To read more individual articles, please click here.
