No System is Perfect
No system is perfect. Just as malfunctions in manufacturing yield defective products, the intricate machinery within cells is susceptible to errors that can trigger system-wide failures. In the cellular context, one loose bolt in cellular machinery can cause the assembly line for protein synthesis to rapidly go astray, with consequences ranging from temporary inefficiencies in cellular functions to the catastrophic event of cell death. The production of proteins is a systematic process, moving from the blueprint encoded in DNA, transcribed into RNA, and finally translated into proteins that serve diverse functions, from catalyzing reactions to maintaining cellular integrity. However, these proteins can get misfolded and interact with other misfolded proteins to form larger structures, called aggregates. The consequences of misfolded proteins are far-reaching, contributing to a plethora of diseases. These include cystic fibrosis, retinal degeneration, and neurodegenerative diseases.2 At the endoplasmic reticulum (ER), misfolded protein aggregates induce cellular stress that contributes to the development of various kidney diseases, such as diabetic nephropathy, renal fibrosis, and ischemia-reperfusion.3 Fortunately, the cell employs quality control mechanisms that regulate and remedy misfolded membrane proteins.
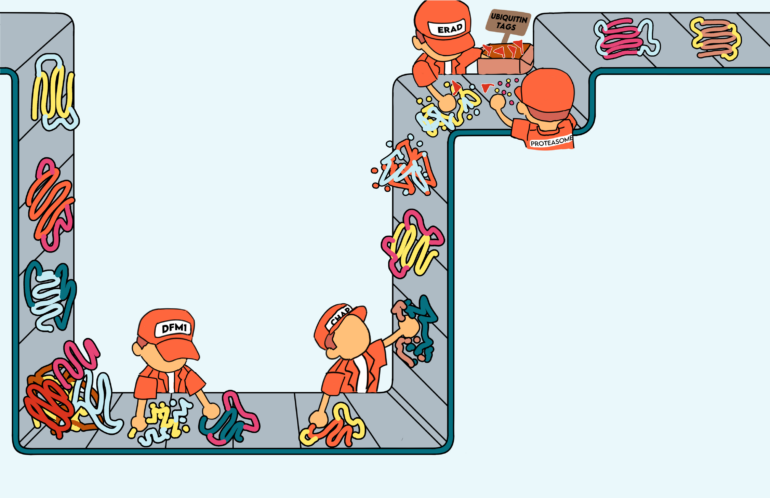
Most membrane proteins are synthesized at the ER, where they undergo cotranslational translocation: the simultaneous translation and transport of proteins across and into the ER membrane.1 However, when proteins get misfolded due to errors with post-translational modifications or improper disulfide bond formation hindering them from achieving stable conformations, they undergo retrotranslocation. This process involves transport from the ER back to the cytosol for degradation.1 This dislocation process relies on the ER-associated degradation (ERAD) pathway, which utilizes a small protein called ubiquitin to tag misfolded proteins for degradation by a large complex of proteases called the proteasome.1 Acting as inspectors to maintain cellular integrity, the proteasome recognizes the ubiquitin tag on defective items in the protein production line and processes them by breaking down these misfolded proteins.
Distinguishing Rhomboid Proteases From Rhomboid Pseudoproteases
Transmembrane proteins are proteins that contain two or more helical membrane segments.4 For over two decades, the exit route to allow retrotranslocation of these misfolded transmembrane proteins from the ER to the cytosol was unknown.5 In 2018, Dr. Sonya Neal, an associate professor of cell and developmental biology at UC San Diego, discovered that the yeast rhomboid pseudoprotease, Derlin Dfm1, was responsible for the retrotranslocation of misfolded membrane proteins in the ERAD pathway.6 Rhomboid proteases are intramembrane proteases that act within the ER membrane and cleave misfolded transmembrane proteins. Their active sites are buried in the lipid bilayer of the membrane and cleave other transmembrane proteins.7 Many rhomboid proteases participate in protein quality control by cleaving misfolded proteins to facilitate their retrotranslocation. Interestingly, there are several rhomboid protease homologs called rhomboid pseudoproteases, including Dfm1, that lack the amino acids essential for catalyzing reactions.8 Without these catalytic components that are crucial for proteolysis, rhomboid pseudoproteases cannot cleave other proteins, thereby spurring investigations of the potential functions of yeast derlin Dfm1.
Guardian of Proteome Homeostasis
Misfolded membrane protein aggregates trigger stress at the ER, which occurs when the capacity of the ER to fold proteins becomes saturated.9 In essence, the accumulation of misfolded proteins jams the gears of the ER machinery, preventing the ER from meeting the demands for protein folding and ultimately causing stress. The constant pressure results in the emergence of various kidney diseases and upregulation of ER-resident chaperones: proteins that work to alleviate stress by assisting in the proper folding or unfolding of other proteins.3,10 To address persistent ER stress, podocytes (specialized kidney cells) employ Derlin-2 as a crucial protein quality control mechanism.3 Notably, mouse models of kidney disease and individuals with diabetic nephropathy exhibit elevated levels of Derlin-2.3 This underlines the significance of Derlin-2 in mitigating ER stress in the context of kidney diseases. Furthermore, derlins play an important role in the regulation of various transmembrane proteins, including potassium channels and the cystic fibrosis transmembrane conductance regulator.3 Rhomboid pseudoproteases can act on a variety of membrane protein substrates, exemplifying their diverse impact on cellular processes associated with misfolded membrane proteins.
Discovering the Dual Role of Dfm1
Although the substrate toxicity assay demonstrated that Dfm1 prevents cell toxicity caused by misfolded membrane protein aggregates, the method by which Dfm1 effectively solubilizes and resolves these aggregates remained unknown. Pulling from a collection of retrotranslocation-defective Dfm1 mutants, Dr. Kandel made a significant discovery on more precise functions of Dfm1. She found that Dfm1 not only assists in the retrotranslocation of misfolded membrane proteins across the ER membrane, but it also acts as a chaperone.2 Dfm1 holds unfolded or partially folded proteins in a stable state, thereby preventing aggregation or misfolding2 (Figure 1). This identification of chaperone-like activity is a pioneering accomplishment, marking the first instance of such activity attributed to any rhomboid protein. To assess the impact of the Dfm1 mutant, Dr. Kandel employed a detergent solubility assay. This assay leverages the distinction between aggregated and nonaggregated misfolded proteins, conveying that aggregated proteins resist detergents, whereas nonaggregated proteins are susceptible to detergent action.5 In the absence of the Dfm1 mutant and the presence of a detergent, Hmg2 was entirely aggregated. Conversely, in the presence of the Dfm1 mutant and a detergent, Hmg2 showed no signs of aggregation.2 These findings pave the way to uncovering how Dfm1 can resolve protein aggregates, separate from its role in the retrotranslocation of proteins in the ERAD pathway. Given the implications of protein aggregation in numerous human diseases, understanding the intricate mechanisms governed by Dfm1 holds great promise for both foundational and translational aspects of cell biology.
The Free Ubiquitin Pool
Interestingly, Dr. Kandel also observed that not all aggregated membrane proteins proved toxic—even in the absence of Dfm1. This prompted her and her colleagues to explore the dynamics of the free ubiquitin pool. Within the ubiquitin-proteasome system, ubiquitin forms polyubiquitin chains when attached to a target protein, signaling the proteasome to recognize and degrade the targeted protein (Figure 2).1 In the cytosol, there exists a pool of ubiquitin molecules in a “free” or unbound state, known as the “free ubiquitin pool.”2 These reusable “ubiquitin tags” mark defective proteins as they roll off the production line. The meticulous regulation of the free ubiquitin pool is integral to cellular protein quality control and maintenance. Dr. Kandel’s investigation led to a crucial discovery: toxicity emerged not only from protein aggregation but also from the sequestration of ubiquitin due to excessive ubiquitination of misfolded membrane proteins. Dr. Kandel hypothesized that this sequestration results in the depletion of the free ubiquitin pool, which increases the tendency of non-ubiquitinated misfolded membrane proteins to aggregate and induce cellular stress.2 Further research is required to investigate this hypothesis. Nevertheless, this finding highlights the intricate interplay between protein aggregation, ubiquitin dynamics, and cellular health.
Regulating Sphingolipid Production
Dfm1 functions with an additional layer of complexity by laying the groundwork for potential breakthroughs in comprehending diseases linked to disrupted sphingolipid metabolism.11 Sphingolipids are a major class of lipids that play important roles in maintaining the structural integrity and function of cellular membranes, including the ER membrane. Sphingolipids are also involved in various cellular processes, including signal transduction, cell adhesion, and apoptosis.12 The preservation of sphingolipid homeostasis, which involves the control of sphingolipid levels, synthesis, and degradation, is crucial for protein synthesis. Any disruption of sphingolipid metabolism negatively impacts the ER protein quality control machinery by inducing ER stress. Consequently, the unfolded protein response becomes activated, which promotes transcription of genes involved in protein folding, degradation of misfolded proteins, and other processes that alleviate ER stress.11,12
Dfm1’s Multifunctional Prowess and the Promise of Therapeutic Innovation
Dfm1 emerges as a key orchestrator in the intricate protein production assembly line within cells. This small but mighty protein takes on multiple roles: facilitating retrotranslocation of misfolded membrane proteins, resolving aggregates by acting as a chaperone, and maintaining the delicate balance in cellular sphingolipid composition. Understanding Dfm1’s multifunctional prowess not only enriches comprehension of cellular dynamics but also unveils promising avenues for therapeutic exploration. Much like optimizing an assembly line for efficiency, the mastery of Dfm1’s roles holds the promise of refining interventions for diseases, including cystic fibrosis, retinal degeneration, neurodegenerative diseases, and kidney diseases. As scientists dive deeper into the intricacies of this cellular production line, they pave the way for innovations that may reshape the landscape of disease treatment. Ultimately, dissecting the nuances of Dfm1’s involvement in ER stress offers hope for future breakthroughs in the quest to improve therapeutic strategies against the repercussions of misfolded membrane proteins.
References
- Lodish HF, Berk A, Kaiser CA, Krieger M, Bretscher A, Ploegh H, Martin KC, Yaffe M, Amon AA. 13.3 Protein Modifications, Folding, and Quality Control in the ER. In: Molecular cell biology. 9th ed. New York, NY: Macmillan International Higher Education; 2022.
- Kandel R, Jung J, Syau D, Kuo T, Songster L, Aguayo A, Duttke S, Benner C, Neal S. Derlin DFM1 employs a chaperone function to resolve misfolded membrane protein stress. 2022. doi:10.1101/2022.01.25.477788
- Kandel RR, Neal SE. The role of rhomboid superfamily members in protein homeostasis: Mechanistic insight and physiological implications. Biochim Biophys Acta Mol Cell Res. 2020;1867(10):118793. doi:10.1016/j.bbamcr.2020.118793
- Smalinskaitė L, Hegde RS. The biogenesis of multipass membrane proteins. Cold Spring Harb Perspect Biol. 2022;15(4). doi:10.1101/cshperspect.a041251
- Kang K, Kandel R. 2023. Saltman Quarterly Journal features article interview on Dfm1 rhomboid pseudoprotease.
- Neal S, Jaeger PA, Duttke SH, Benner C, Glass CK, Ideker T, Hampton RY. The DFM1 derlin is required for ERAD retrotranslocation of integral membrane proteins. Molecular Cell. 2018;69(2). doi:10.1016/j.molcel.2017.12.012
- Brown MS, Ye J, Rawson RB, Goldstein JL. Regulated intramembrane proteolysis. Cell. 2000;100(4):391–398. doi:10.1016/s0092-8674(00)80675-3
- Düsterhöft S, Künzel U, Freeman M. Rhomboid proteases in human disease: Mechanisms and future prospects. Biochim Biophys Acta Mol Cell Res. 2017;1864(11):2200–2209. doi:10.1016/j.bbamcr.2017.04.016
- Lin JH, Walter P, Yen TSB. Endoplasmic Reticulum stress in disease pathogenesis. Annu Rev Pathol. 2008;3(1):399–425. doi:10.1146/annurev.pathmechdis.3.121806.151434
- Welihinda AA, Tirasophon W, Kaufman RJ. The cellular response to protein misfolding in the endoplasmic reticulum. Gene Expression. 2018;7.
- Bhaduri S, Aguayo A, Ohno Y, Proietto M, Jung J, Wang I, Kandel R, Singh N, Ibrahim I, Fulzele A, et al. An ERAD‐independent role for Rhomboid pseudoprotease DFM1 in mediating sphingolipid homeostasis. EMBO J. 2022;42(4). doi:10.15252/embj.2022112275
- Breslow DK. Sphingolipid homeostasis in the endoplasmic reticulum and beyond. Cold Spring Harb Perspect Biol. 2013;5(4). doi:10.1101/cshperspect.a013326
- Han S, Lone MA, Schneiter R, Chang A. ORM1 and orm2 are conserved endoplasmic reticulum membrane proteins regulating lipid homeostasis and protein quality control. Proceedings of the National Academy of Sciences. 2010;107(13):5851–5856. doi:10.1073/pnas.0911617107
- Davis D, Kannan M, Wattenberg B. Orm/ORMDL Proteins: Gate guardians and master regulators. Advances in Biological Regulation. 2018;70:3–18. doi:10.1016/j.jbior.2018.08.002