BACKGROUND
Gene therapy has grown in popularity as a potential treatment strategy for a variety of human diseases.¹ Adeno-associated virus (AAV) vectors have been shown to be effective gene delivery candidates based on their safety and ability to sustain expression of a transfected gene over extended time periods in the brain.¹ In particular, the AAV-PhP.eB viral capsid vector is known to be effective at transducing the central nervous system (CNS) in mice when delivered intravenously (IV).² However, more research is needed to identify cell-specific promoters that can target gene expression to specific brain regions. In this study, we investigated whether using a 2.2kb Ple394 promoter in the AAV-PhP.eB vector influences the specificity of expression of an eGFP transgene to a specific region of the brain. The Ple394 promoter derives its name from the Pleiades Promoter Project, which developed an array of promoters as tools to enhance the study of gene therapies and targeted gene expression.³
METHODS
One mouse received 150μL of phosphate-buffered saline (PBS) as a negative control. Four mice received 3×1010 vector genomes (vg) of PhP.eB-CAG-eGFP as positive control viral capsid. The CAG promoter is a pancellular chicken beta-actin promoter, which is known to be a ubiquitous promoter and is expressed in most mouse tissues. Eight additional mice received PhP.eB-Ple394-eGFP vectors as the treatment group. Of these eight, three received 6.25×1010 viral genomes (low dose), three received 1.25×1011 viral genomes (medium dose), and two received 2.5×1011 viral genomes (high dose). Vector was delivered in 150μL intravenous injections. A waiting period of four weeks followed. The animals were perfused with phosphate-buffered saline, euthanized, and dissected. The brains were fixed with paraformaldehyde and sagittally sectioned using a sliding microtome to give sections of 40 μm thickness. Immunohistochemistry was performed using DAB-labeling with rabbit anti-eGFP and Donkey anti-Rabbit antibodies, and the sections were viewed with an Axioscan microscope at 10x.
RESULTS
Immunostain results showed that the ubiquitously expressed PhP.eB-CAG-eGFP vector transduced both neurons and astroglia throughout the brain (Figure 1). Transduced neurons labeled by eGFP presented as small cell bodies with extended axonal and dendritic processes, while astroglia presented as roughly circular regions of diffuse processes. In contrast, transfection with the vector containing the Ple394 promoter showed almost no gene expression in astroglia and non-cortical brain regions such as striatum and thalamus. However, at all vector concentrations, the Ple394 promoter caused strong GFP expression in the cortical regions such as the prefrontal cortex. Only at the very highest concentration did this vector also show expression in the brainstem (Figure 1). Overall, the Ple394 images reveal strong transduction of cortical neurons and markedly reduced expression in non-cortical areas.
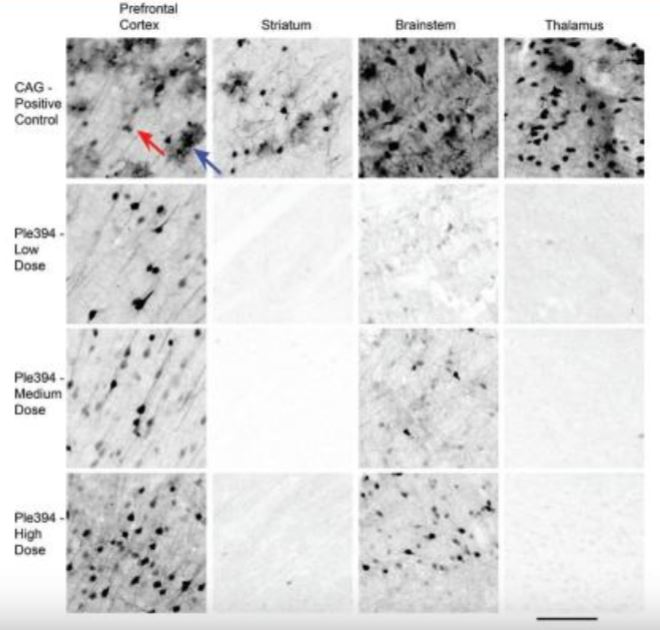
An immunostain against eGFP was done on 40μm thick sagittal brain sections from mice that received intravenous injections of either AAV-PhP.eB-CAG-eGFP (CAG-Positive Control) or AAV-PhP.eB-Ple394-eGFP at low (Ple394-Low Dose), medium (Ple394-Medium Dose), or high dosages (Ple394-High Dose). The brain sections are roughly 1.4 mm from midline. Prefrontal cortex is an on-target area. Striatum, brainstem, and thalamus are off-target areas. Red arrow indicates an eGFP stained neuron. Blue arrow indicates an eGFP stained astroglial cell. Scale bar: 100μm
DISCUSSION
Our experiment showed that the Ple394 promoter expressed strongly in cortical neurons and eliminated gene expression in regions such as the thalamus and striatum. However, some off-target expression remained in regions such as the brainstem. In the future, more research with larger samples will be needed to determine whether this dramatic effect is fully reproducible and whether the limited amount of off-target expression is biologically relevant or deleterious. Ultimately, we would wish to determine whether the Ple394 promoter can be used to treat defects in human cortical gene expression such as those found in specific human neurological diseases.
REFERENCES
- Dunbar, C. E. et al. “Gene therapy comes of age.” Science 359, no. 6372 (2018).
- Chan, K.Y. et al. “Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems.” Nature Neuroscience 20, no. 8, 1172-1179 (2017).
- Portales-Casamar, E. et al. “A regulatory toolbox of MiniPromoters to drive selective expression in the brain.” Proceedings of the National Academy of Sciences 107, no. 38, 16589-16594 (2010)
WRITTEN BY BRYAN CHAVEZ
M.S. Biology, UC San Diego 2021, Tuszynski Lab, Translational Neuroscience Institute
FROM SALTMAN QUARTERLY VOL. 18
To read the original version, please click here. To read the full version on our website, please click here. To read more individual articles, please click here.