No cancer is desirable; however, a glance at the statistics is enough to suggest that a diagnosis of ovarian cancer is particularly unfortunate. In 2018, 184,799 deaths occurred due to ovarian cancer, accounting for 4.4% of cancer-related mortality among women. Around 90% of people with ovarian cancer will survive for at least five years after a diagnosis. Such a low survival rate provides a compelling argument for substantial investment in ovarian cancer research. Even so, the disease went unnoticed. Between 2007 and 2014, the US National Cancer Institute provided about 19 times more funding for breast cancer research than it did for the study of ovarian cancer research.
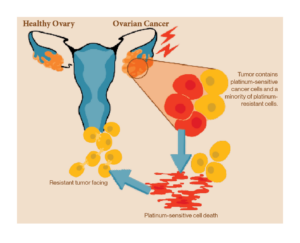
Nearly a quarter of a million women are diagnosed with ovarian cancer each year. More than half will die within five years of getting diagnosed. Most patients who die because of their cancer develop resistance to the most common form of treatment—platinum-based chemotherapy. The platinum treatment damage the DNA of cancer cells, triggering programmed cell death via apoptosis. Most patients respond well to treatment initially, but many relapse within 18 months. If ovarian cancer recurs within six months of chemotherapy, it is considered resistant, and subsequent treatment will rely on non-platinum-based options. As a result, almost all patients will eventually relapse with a tumor that has developed resistance to platinum-based therapy. Platinum-resistant tumor cells use a variety of techniques to fight back the platinum treatment. Some resistant cells learn to repair damaged tumor DNA, while others pump out platinum using transport channels before it can do any damage. Many ovarian cancer researchers are utilizing their limited funds to find ways to overcome this resistance. One approach is to block the mechanisms that cells use to repair tumor DNA damage with drugs such as Poly(ADP-ribose) polymerase (PARP) inhibitors.
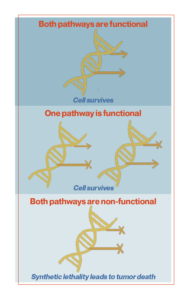
PARP inhibitors are a class of drugs that researchers are utilizing to fight drug-resistant cancer cells. These inhibitors use a principle known as synthetic lethality. Synthetic lethality represents a type of lethal cellular interaction in which the simultaneous occurrence of two events leads to cell death. Redundant signaling pathways allow cells or organisms to continue to function and reproduce the impairment of key signaling pathways. Cells can tolerate the loss of a single gene in isolation, but not the combined loss of multiple overlapping genes or signaling pathways within the cell. Synthetic lethality occurs when both primary or redundant genes or pathways are disabled. Blocking just one of these pathways may not be lethal, but a cell cannot survive if it loses both.
Cancer cells often accumulate mutations, especially in signaling pathways that involve DNA repair. It is not always clear whether these defects are the cause or consequence of tumorigenesis (the growth of malignant properties of normal cells), but they are often present when the patient is first diagnosed. Cancer-associated mutations in DNA repair proteins make cancer cells dependent on alternative repair pathways. Therefore, these tumor-associated mutations provide an initial pathway block and cancer cells can be killed by targeting alternative pathways.
The synthetic lethality of PARP inhibitors is a simple mechanism. PARP proteins are involved in DNA break repair. PARP inhibitors block the PARP protein from repairing single-strand breaks, exacerbating DNA damage and causing double-strand breaks. In most cells, another mechanism for repairing double-strand breaks, known as homologous recombination, can repair and save the cell. But in some cancer cells, this second line of defense is also compromised. This is a condition called homologous recombination deficiency (HRD). About half of ovarian cancers have HRD, and about one-fifth of the exhibiting group are due to mutations in the BRCA (breast cancer) gene. BRCA genes encode proteins involved in DNA repair. Essentially, BRCA-mutant cells are unable to repair damage caused by PARP inhibitors. Tumor cells exhibiting HRD, therefore, fail to completely repair cancerous DNA, and the accumulation of DNA damage increases genomic instability, ultimately leading to cell death.
There are currently three FDA-approved PARP inhibitors for ovarian cancer: olaparib, niraparib, and rucaparib. Olaparib is the most potent PARP inhibitor to date. It has been very successful in many Phase I and Phase II trials in BRCA-associated ovarian cancer. A landmark clinical trial, called SOLO-1, testing olaparib as first-line maintenance therapy in patients with BRCA mutations, has published notable results. Olaparib was observed to increase tumor-free survival by 3 years compared to patients who received a placebo. Nearly half of the patients treated with ovarian cancer were still ovarian cancer-free after five years, compared with only 20% of placebo patients.
The researchers’ next goal was to investigate whether PARP inhibitors would benefit patients without BRCA mutations. To test this, we designed a study in which PARP inhibitors were administered alone or in combination with other therapies, regardless of BRCA mutation status. This study included his three studies of PAOLA-1, PRIMA, and VELIA. The first study, called PAOLA-1, combined olaparib with bevacizumab, an antiangiogenic drug that depletes tumors of oxygen-supplying vessels. The PRIMA trial tested niraparib in patients at high risk of recurrent disease. The VELIA trial tested veliparib alongside chemotherapy. Veliparib has slightly different side effects than his other PARP inhibitors, including less blood cell suppression. This means that, unlike others, it can be used in conjunction with chemotherapy. All three studies showed significantly improved progression-free survival in all-treated patients compared with placebo patients. This strongly supports the use of PARP inhibitors as first-line therapy.
Although PARP inhibitors can be used to treat resistant tumors, it remains difficult for clinicians to predict whether a patient will be platinum-resistant. Non-platinum-based therapies are used to treat patients because they do not work. However, a group of researchers discovered a protein called RAD51 that is involved in homologous recombination. The team measured the RAD51 levels in tissue samples from people who received only platinum-based chemotherapy as first-line therapy. They found that people with the lowest RAD51 scores had better outcomes and later experienced cancer recurrence than those with the highest levels. Since the tumor samples were exposed to platinum, researchers hypothesize that low RAD51 activity may indicate a lower likelihood for the development of platinum resistance. Clinicians may use RAD51 when deciding whether to combine another drug with platinum-based chemotherapy during the first round of treatment, start a second maintenance drug once chemotherapy is complete, or encourage a patient to enroll in a clinical trial.
Platinum resistance is difficult to predict and treat as it has no single cause. In addition to enhanced DNA repair mechanisms, tumor cells can speed processes that improve cell survival, generate higher levels of molecular pumps to evade drugs and produce proteins that remove platinum from the genome. may generate Any combination of these mechanisms that may exist within an individual, and decades of clinical trials have tested drugs that target them individually. However, since ovarian tumors are constantly adapting, it is not enough to pursue one resistance mechanism. Therefore, approaches that approach platinum-resistant tumors from multiple perspectives are the most promising.
In a 2014 study, researchers combined paclitaxel with a monoclonal antibody called bevacizumab, which blocks blood vessel formation by inhibiting vascular endothelial growth factor (VEGF). This combination increased the survival time of people with platinum-resistant tumors by three months compared with those who received chemotherapy alone. Although the mechanism of antibody function is unknown, combination therapy is the standard for clinicians.
Laboratory studies showing that VEGF inhibitors reduce BRCA and RAD51 protein production have led some research groups to combine these compounds with PARP inhibitors, with the hope that supplementation would make Platinum-resistant cancer cells more sensitive to PARP inhibition. When prescribed alone, PARP inhibitors shrink tumors in 25% of platinum-resistant patients with BRCA mutations and 5% in those without BRCA mutation.
A combination approach could also allow immunotherapy drugs to work against platinum-resistant ovarian cancer. The researchers are interested in how proteins activated by DNA damage interact with the immune system. Exploring the immune system’s response is essential because DNA repair can be affected by how immune cells recognize damaged DNA. If the immune system recognizes damaged DNA as a threat, cancer cells can be eliminated. In cell line and mouse experiments, cumulative DNA damage in the presence of PARP inhibitors prompts tumor cells to produce higher levels of the immune checkpoint protein PD-L1, a T-cell inhibitor. T cells circulate in the body and destroy infected host cells. In this case, the cancer cell can be exposed to killer T cells by reducing PD-L1. This leads to a strategy of inactivating the effects of PD-L1 that may offer a better opportunity for PARP inhibitors in platinum-resistant cancer.
Other interesting strategies are also being explored by researchers. When a cell detects DNA damage, it activates a sequence of proteins that stop cell division. Once the cell stops dividing, it allows the cell to repair its DNA before attempting to copy it. Inhibition of these proteins with a drug removes this division-stopping checkpoint and the tumor cell resumes before repair can be made. This can lead to cells clogging and dying as they try to divide or transmit DNA damage that would weaken new tumor cells. In advanced ovarian cancer, one of these checkpoints is already damaged, so researchers are taking advantage of this phenomenon. It is very beneficial for the patient if the tumor weakens on its own without the need for chemotherapy which often has serious negative side effects.
These types of personalized, targeted approaches are likely to become more common as researchers study the biology of platinum resistance. Drugs like paclitaxel and VEGF inhibitors have made a huge difference for thousands of women with platinum-resistant ovarian cancer. It is hoped that a better understanding of the disease will lead researchers to the next big steps. The researchers hope to rewire the immune system to overcome platinum resistance, and vaccine trials are underway that aim to trick immune cells into recognizing and attacking the cells. cancer cells. After decades of limited improvement, there is finally a sense of optimism. The growing understanding of ovarian cancer and the mechanisms of resistance to treatment could soon translate into real benefits for women.
Sources:
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6500433/
- https://www.cancer.gov/about-nci/budget/fact-book/data/research-funding
- https://www.sciencedirect.com/science/article/pii/S1044579X20301930
- https://www.annualreviews.org/doi/10.1146/annurev-cancerbio-042016-073434
- https://www.oncotarget.com/article/8196/text/
- https://pubmed.ncbi.nlm.nih.gov/33004253/
- https://www.nature.com/articles/s12276-021-00557-3
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4771499/
- https://pubmed.ncbi.nlm.nih.gov/30345884/
- https://www.annalsofoncology.org/article/S0923-7534(20)40946-9/fulltext
- https://pubmed.ncbi.nlm.nih.gov/31851799/
- https://www.nejm.org/doi/10.1056/NEJMoa1910962
- https://www.nejm.org/doi/10.1056/NEJMoa1909707
- https://pubmed.ncbi.nlm.nih.gov/33709473/
- https://pubmed.ncbi.nlm.nih.gov/15367671/
- https://pubmed.ncbi.nlm.nih.gov/24637997/
- https://pubmed.ncbi.nlm.nih.gov/16357170/
- https://www.gynecologiconcology-online.net/article/S0090-8258(16)30065-8/fulltext