Tom Patterson was the first patient in the United States to receive an intravenous dose of viruses as a cure for the bacterial infection that put him in a coma. Now, you might be overcome with curiosity when reading that sentence. You might also be wondering, Why do we need viruses to kill bacteria when we already have antibiotics? Antibiotics have been used for decades in medicine, agriculture, ethanol production, and countless other fields. In the meat industry, antibiotics are mass-fed to pigs, cattle, and chicken to make them grow fatter. In medicine, antibiotics are prescribed for any and every bacterial inconvenience. When bacteria develop resistances, scientists are quick to engineer new antibiotics. However, there is only a finite number of antibiotics we can make, and we have reached an unbreakable threshold: our current resources prevent us from discovering and designing effective antibiotics. Thus, what started out as a promising weapon against monstrous bacteria has turned into a dystopian story about antibiotic resistance and superbugs. Antibiotic resistance occurs when bacteria mutate and develop the ability to defeat the drugs that were engineered to kill them. This phenomenon started occurring shortly after the overuse of antibiotics, creating superbugs that are immune to the effects of all existing antibiotics and deadlier than ever. Hence, we had no cure for the types of diseases these super-bacteria spread, at least until we discovered the magnificence of phage therapy.
Dr. Tom Patterson, professor of psychiatry at UC San Diego School of Medicine, was infected by bacterium Acinetobacter baumannii, a superbug on the WHO’s list of the most deadly pathogens in the world. Acinetobacter baumannii is multi-drug resistant bacteria – a bacterial kleptomaniac that steals antimicrobial resistance genes from other bacteria. This infection caused Dr. Patterson to develop an abscess the size of a football in his abdomen and made him comatose. In her quest for a cure, his wife Dr. Steffanie A. Strathdee, the Associate Dean of Global Health Sciences at UC San Diego School of Medicine, found a paper that explored phage therapy.
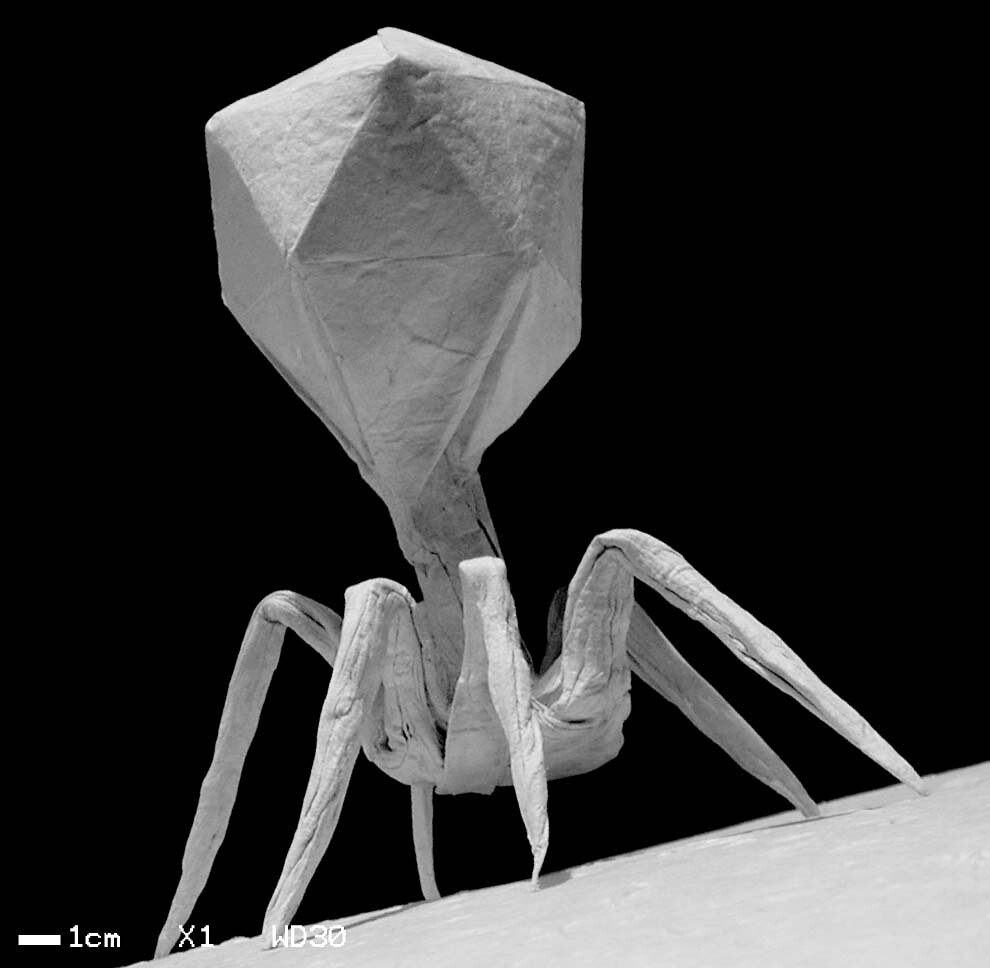
Phage, short for bacteriophage, is a virus that naturally evolved to attack bacteria. They are more abundant than all organisms on Earth combined and commit genocide for a living. Discovered by Félix d’Hérelle in 1917 and first seen under the electron microscope in 1940, bacteriophages are highly specialized viruses that target and kill one specific bacteria (and some of its very close relatives). So how does a phage that is 100 times smaller than its prey manage to infiltrate and overtake the bacteria? Once a phage identifies its specific bacteria, it connects its tail fibers with the receptors on the bacteria’s surface and punctures this surface with a syringe-like protrusion. The phage then injects its genetic information into the bacteria, which replicates multiple times, producing numerous progeny of itself. These progeny then produce an enzyme called endolysin, which punches a hole in the bacteria at high pressures, resulting in bacterial cell death.
If phages are bacteria specific, how can we know which phage corresponds to which bacteria? We need to perform a procedure known as a plaque assay in which phages are placed on bacterial colonies growing on an agar plate and kept aside. After 24 hours, Dr. Strathdee describes that the agar should look like swiss cheese if there are active phages present against that specific bacterial colony.
So, aside from the obvious reason, why are phages better than antibiotics at targeting and obliterating bacteria? When phages are intravenously administered, we inject a drug unlike others: a drug that is alive. Upon entry into the human body, phages are constantly multiplying, which means that the actual dosage of the drug received is many times higher than the dosage administered. Furthermore, antibiotic treatment has been found to alter the infection microenvironment, affecting the target bacteria, immune cells, and healthy tissue. Thus, one can compare the two treatments where phage therapy acts like a targetted missile as opposed to antibiotic treatment which acts more like a nuclear bomb.
Additionally, phages are also emerging effective in anti-cancer research. Given the specific nature of phages, they can be used as a vehicle to deliver cancer-fighting treatment directly to cancer cells. They can also be used to identify molecular determinants of cancer cells. Moreover, scientists are theorizing and researching the manipulation of the phage’s genetic material to transport “suicide genes” inside cancer cells themselves!
Now, you may be thinking that if bacteria can develop resistance to antibiotics, can they also develop a resistance to phages? Although viruses naturally evolved to attack bacteria, phage resistance in bacteria is a very real possibility. However, in order for bacteria to develop phage resistance, they would have to lose resistance to antibiotics. Thus, humans remain void from deadly bacterial infections.
Dr. Patterson eventually woke up from his coma and made a complete recovery after receiving the first ever intravenous phage therapy. If you wish to know more about his journey with phage therapy, check out “The Perfect Predator” by Steffanie Strathdee & Tom Patterson. Today, Dr. Patterson and Dr. Strathedee have led efforts to normalize phage therapy as a primary form of treatment in the case of bacterial infections. Five patients in UC San Diego Health have undergone phage therapy; one of these patients recovered from a chronic infection and underwent life-saving heart transplant surgery. Furthermore, the FDA has approved clinical trials for intravenous phage therapy upon which the future of this extraordinary treatment rests.
References
- https://www.youtube.com/watch?v=0rrHGlqeD4g
- https://youtu.be/YI3tsmFsrOg
- https://wyss.harvard.edu/news/antibiotics-alter-the-infectious-microenvironment-and-may-reduce-the-ability-of-immune-cells-to-kill-bacteria/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7923149/
- https://health.ucsd.edu/news/topics/phage-therapy/pages/default.aspx
- https://www.cdc.gov/antibiotic-use/q-a.html