In 2019, immunologist Leslie Norins offered to award one million dollars of his own money to any scientist who could prove that Alzheimer’s disease was caused by a germ. Alzheimer’s is a progressive neurodegenerative disease that accounts for 60-80% of dementia cases, and the theory that an infection might cause Alzheimer’s has been lurking at the periphery of neuroscience research for decades. However, unlike Norins, the majority of Alzheimer’s researchers think that the key culprits are sticky molecules in the brain called amyloids. The amyloid hypothesis holds that Alzheimer’s results from a build-up of sticky, soluble proteins–amyloid-β peptides–in the spaces between brain cells. These peptides are cleaved by another protein embedded in the membranes of neurons. Once freed, the peptides clump together and aggregate into plaques. The plaques then trigger a deadly cascade provoking neuroinflammation and spawning bundles of stringy proteins called tau tangles. Neurons subsequently perish under such duress. The amyloid theory is believed by many scientists; however, recent research has provided intriguing hints that suggest infection could seed some cases of Alzheimer’s disease by triggering the production of amyloid clumps.
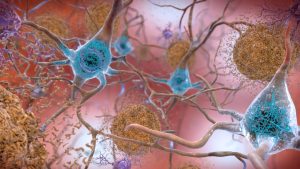
The data hint at a radical role of amyloids in neurons. Instead of just being a toxic waste product, amyloids might have an important job of their own–helping to protect the brain from infection. However, various factors like age or genetics can turn the amyloid from a defender into a villain. Researchers hoping to test the infection hypothesis have gone hunting for microbes in the post-mortem brains of thousands of Alzheimer’s patients and even found them in many.
Ruth Itzhaki, a biophysicist at the University of Manchester, reported observations of herpes simplex virus 1 (HSV1) in post-mortem Alzheimer’s brains in the 1990s. Itzhaki believes that the presence of microbes in the brain must indicate a function for them in the brain, a hypothesis which she and many others think provides solid evidence to support that viruses are a keystone in Alzheimer’s.
Several microbes have been proposed as triggers of Alzheimer’s, including three human herpes viruses and three bacteria. Most research groups in this field have a favored microbe and two prominent papers in 2018 examined the role of herpes viruses. One of them was from the group of Joel Dudley at the Icahn School of Medicine at Mount Sinai. They analyzed huge chunks of data on genes, proteins, and tissue structure generated from nearly 1,000 post-mortem brains available in various databases in search of telltale signatures of viruses in brain tissue–snippets of genes or proteins specific to herpes–and concluded that levels of human herpesvirus 6A (HHV-6A) and human herpesvirus 7 were higher in people who had Alzheimer’s disease than in controls.
However, other researchers, like virologist Steven Jacobson at the National Institute of Neurological Disorders and Stroke, whose team studied a sample of more than 1,000 post-mortem brains, failed to replicate Dudley’s findings. Furthermore, despite the impressive number of individual brains in Dudley’s study, the results are only correlative at best.
When human studies provide only correlation, researchers often turn to animal experiments to look for the cause. However, animal models of Alzheimer’s aren’t perfect. Mice, for instance, do not develop the hallmark plaques as they age, unless they are genetically engineered to produce them. Nevertheless, the widely used 5xFAD transgenic mouse expresses five relevant mutations in genes that code for the pre-amyloid protein and one of the enzymes that chop it into amyloid-β. These mice express the genes at high levels, developing plaques when they are only two months old. Neurogeneticist Rudolph Tanzi and his colleagues at Massachusetts General Hospital used 5xFAD mice to identify Alzheimer’s risk genes from new human genomics data. He was puzzled to see a gene for CD33, a protein widely expressed in the innate immune system, show up in the genomics data. He then tested to see if amyloid-β could be an antimicrobial peptide by experimenting if amyloid-β could kill eight common disease-causing microorganisms, including Streptococcus pneumoniae and Escherichia coli, and concluded that it could act as an antimicrobial peptide, forming the basis of the antimicrobial protection hypothesis. They injected the bacterium Salmonella typhimurium directly into the brains of plaque-making 5xFAD mice and found that they survived longer than non-transgenic, plaque-less mice. They found similar results in nematode worms, using the pathogenic fungus Candida albicans. In both cases, amyloids formed sticky nets that engulfed and disarmed the pathogens.
Then the team turned its attention to herpes viruses, which had emerged as the human pathogens most frequently linked with Alzheimer’s disease. They injected HSV1 into the brains of young 5xFAD mice and normal mice. Within three weeks, the brains of the transgenic mice were dotted with amyloid plaques. When the team repeated the experiment with a lethal dose of HSV1, the transgenic mice lived longer than the controls–and plaques appeared in their brains within a remarkable two days!
Tanzi went on to investigate the impact of herpes viruses on tau-tangle formation in the models and whether these tangles could block the spread of viruses down neurons. Aware that whatever is responsible for the onset of the disease might no longer be around by the time the person dies, Tanzi’s laboratory is developing techniques to isolate and analyze individual plaques to see if traces of the microbe are trapped inside, as a sort of archaeological dig.
The upshot of his proof-of-concept experiments so far is that “if you are making amyloid-β, you survive infection better.” But he admits that actual proof–seeing an infection trigger the amyloid cascade to cause Alzheimer’s–is a long way off. Moreover, no one knows yet whether amyloid-β’s antimicrobial properties are deployed as part of a normal physiological process, or how significant they would be in the general palette of defense mechanisms in the brain. An infection could be one way of striking the match that leads to the fire of Alzheimer’s.
As for Norins’s germ quest, eight applicants submitted a formal entry in which six different microorganisms were nominated. Although no one convincingly proved that their nominee was the sole microbe triggering Alzheimer’s, Norins’ quest changed the direction of research from the amyloid hypothesis to the infection hypothesis. That being said, the quest continues.
References:
- https://alzgerm.org/whitepaper-executive-summary/
- https://www.alz.org/alzheimers-dementia/what-is-alzheimers
- https://www.sciencedirect.com/science/article/pii/S0092867405001522
- https://www.sciencedirect.com/science/article/pii/S155252601833228X#sec11
- https://onlinelibrary.wiley.com/doi/abs/10.1002/jmv.1890330403
- https://www.sciencedirect.com/science/article/pii/S0896627318304215
- https://www.sciencedirect.com/science/article/pii/S0896627319310979
- https://stm.sciencemag.org/content/8/340/340ra72?source=post_page—————————
- https://www.sciencedirect.com/science/article/pii/S0896627318305269
- https://www.prnewswire.com/news-releases/nobody-finds-the-alzheimers-germ-in-1-million-challenge-but-eight-researchers-split-200k-says-dr-leslie-norins-of-alzheimers-germ-quest-301232177.html