BY LAUREN BRUMAGE | SQ ONLINE WRITER | SQ ONLINE (2017-18)
Evolutionary biologist Theodosius Dobzhansky once said that “nothing in biology makes sense except in the light of evolution.” That being said, our understanding of the molecular mechanisms through which evolution operates is still incomplete. One UC San Diego lab, headed by Dr. Justin Meyer, is seeking to fill some of the gaps in our knowledge by studying experimental viral evolution [1].
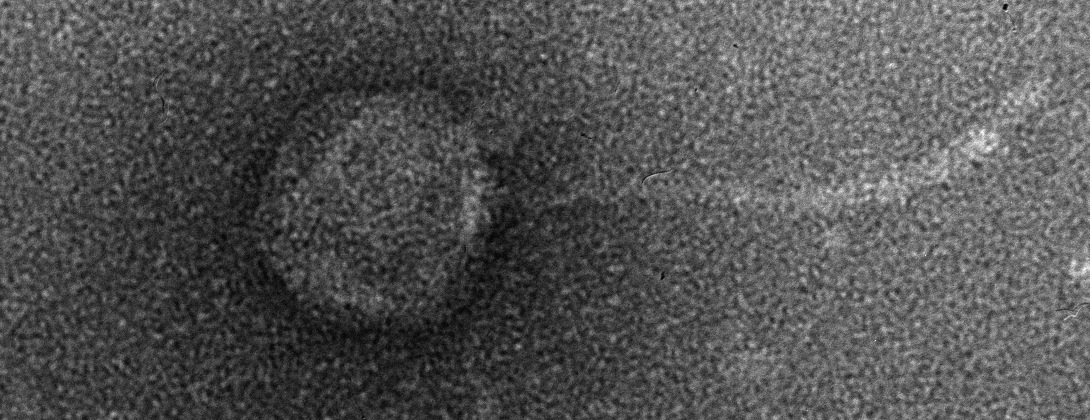
In a recent paper published in Science, lead author Dr. Katie Petrie and other researchers at the Meyer lab elucidate a novel mechanism of evolution in viruses. The focus of their study is bacteriophage λ, a virus that infects Escherichia coli. Phage λ typically exploits host receptor protein LamB for infection, but previous research has shown that the phage can also evolve to use receptor OmpF. Petrie’s Science paper reveals that phage λ’s ability to exploit this new receptor is the result of several mutations in the host-recognition protein that destabilize it. This destabilized protein can then fold differently, allowing it to exploit OmpF in addition to LamB. As a result, phage λ becomes a more flexible, or generalist, virus [2]. Mutations that allow a virus to exploit more than one receptor could, in some circumstances, allow it to infect other hosts. Host range expansion is a critical component of the so-called spillover events that are often linked to emerging infectious diseases.
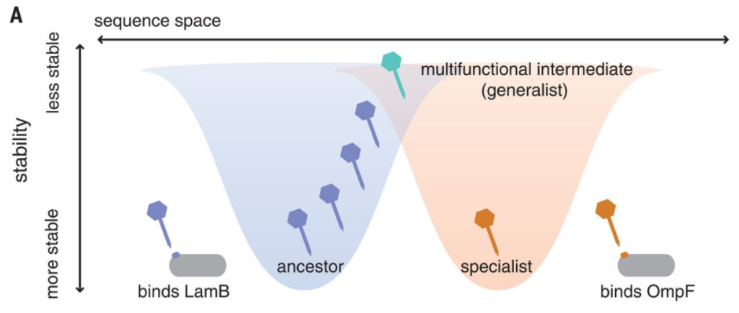
Thus, under a single genotype, phage λ’s host-recognition protein can adopt different conformations in a seemingly random manner. Generalist phage λ particles can then continue evolution under various selection pressures to become specialists that use either OmpF or LamB [2]. Natural selection acts on phenotypic variation to alter the frequency of the underlying genetic variants. However, in this case the variation is entirely non-genetic as the mutations merely cause instability in protein folding leading to random phenotypic variation. In other words, the variation provides the phage flexibility to evolve toward one or the other receptor. This could help the virus persist if it loses the ability to exploit one receptor.
According to Dr. Petrie, the idea that non-genetic (purely phenotypic) variation can drive evolution via natural selection is not new; in fact, the idea dates back to the 1950s or earlier [3]. This study represents the first experimentally observed instance of the phenomenon and the first thorough explanation of how this mechanism of evolution can operate. Dr. Meyer and Dr. Petrie both indicated that they feel the concept of natural selection operating on non-genetic variation may be more widespread than previously thought, so there may be many more examples waiting to be discovered. Moving forward, they plan to look for examples of destabilizing mutations driving evolution in other viruses and to further explore how proteins can evolve new functions via this mechanism [3].
Understanding the processes underlying viral evolution is critical if we wish to stay ahead of potential viral pathogens. Dr. Petrie and Dr. Meyer discussed the possibility of using their findings as part of a surveillance program against infectious diseases caused by viruses when asked about the broader implications of their research on phage λ evolution [3]. According to their Science paper, viruses seem to be more vulnerable to temperature when destabilized, and destabilization seems to be a precursor to exploitation of a novel receptor [2]. Dr. Meyer and Dr. Petrie suggested that sampling viruses from the environment in order to determine their decay rate and temperature sensitivity could perhaps indicate when a virus is undergoing a transition to heightened infectivity or use of alternate receptors [3].
In particular, they cited avian flu as a potential target for this form of surveillance. While avian flu is adapted to infect birds, it is not adapted enough to humans to cause extensive human-to-human transmission. However, current data suggest that it would not take many mutations for a more easily transmissible strain of avian flu to emerge [3, 4]. Avian flu can be devastating in bird populations including in agricultural settings. In some cases, though, avian flu is asymptomatic in birds. Under these circumstances, farmers might unknowingly handle infected poultry. It is therefore important to maintain surveillance of poultry populations to determine what viruses are present and how they might be changing.
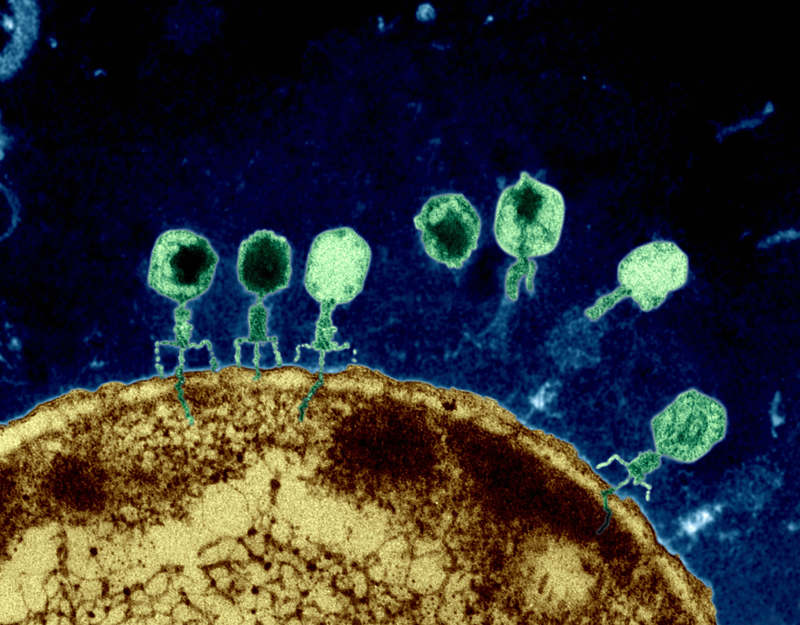
Dr. Petrie and Dr. Meyer are performing groundbreaking research in viral evolution, so I was curious to find out what developments in the study of bacteriophage they are most excited about. They mentioned two subjects of research here at UC San Diego: phage therapy and research on the phage nucleus [3]. The former topic refers to the use of phage, viruses that infect bacteria, to treat infections. Phage therapy has been used at UC San Diego’s Thornton Hospital to effectively treat otherwise incurable antibiotic-resistant infections [5]. The latter topic referenced by Dr. Meyer and Dr. Petrie, the phage nucleus, refers to recent research done by Dr. Joe Pogliano’s lab that supports the hypothesis of viral eukaryogenesis [6]. Essentially, this hypothesis posits that infection by phage contributed to the formation of a nucleus, thereby giving rise to eukaryotic life as we know it.
UC San Diego is a fantastic school for aspiring researchers with many opportunities to become involved in innovative research. At the end of the interview, Dr. Petrie and Dr. Meyer discussed what advice they would give to undergraduates eager to pursue a career in research. Their advice was to join a lab to start getting experience as early as possible and to seek scholarships for summer research [3]. Researching over the summer is a great way to learn what it really means to be a scientist and to be productive in the lab without having to worry about classes. For students seeking a lab position, Dr. Meyer and Dr. Petrie suggested emailing several faculty members directly rather than looking through formal postings [3]. Many labs do not formally post openings but are still eager to take in undergraduate researchers, especially those who show clear enthusiasm for science. Take the initiative to reach out to labs, be persistent, and pursue the research that ignites your curiosity!
[hr gap=”0″]
Sources
- Meyer, J. R. (n.d.). Meyer Lab – Research. Retrieved April 10, 2018, from http://labs.biology.ucsd.edu/meyer/research.html
- Petrie, K. L., Palmer, N. D., Johnson, D. T., Medina, S. J., Yan, S. J., Li, V., . . . Meyer, J. R. (2018). Destabilizing mutations encode nongenetic variation that drives evolutionary innovation. Science,359(6383), 1542-1545. doi:10.1126/science.aar1954
- Petrie, K. L., & Meyer, J. R. (2018, April 13). [Personal interview].
- Butler, D. (2013, April 15). H7N9 bird flu poised to spread. Retrieved from https://www.nature.com/news/h7n9-bird-flu-poised-to-spread-1.12801
- LaFee, S., & Buschman, H. (2017, April 25). Novel Phage Therapy Saves Patient with Multidrug-Resistant Bacterial Infection. Retrieved from https://health.ucsd.edu/news/releases/Pages/2017-04-25-novel-phage-therapy-saves-patient-with-multidrug-resistant-bacterial-infection.aspx
- Chaikeeratisak, V., Nguyen, K., Egan, M. E., Erb, M. L., Vavilina, A., & Pogliano, J. (2017). The Phage Nucleus and Tubulin Spindle Are Conserved among Large Pseudomonas Phages. Cell Reports,(20), 1563-1571. doi:DOI: https://doi.org/10.1016/j.celrep.2017.07.064