BY LAUREN BRUMAGE | SQ ONLINE WRITER | SQ ONLINE (2017-18)
Cancer is a subversive illness that corrupts the body’s own cells, turning them into ever-replicating agents of disease that are nonetheless frequently recognized by the immune system as “self”. This renders it difficult for the body’s immune system to ward off cancer – unless cells from a patient’s body can somehow be trained to seek and destroy cancer cells. At one time, this concept of immunotherapy may have seemed like an insurmountable task born out of a science fiction novel. Now, however, it is a FDA-approved cancer treatment that goes by the name of CAR-T cell therapy.
CAR-T cell therapy is often referred to as a “living drug” treatment as the patient’s own T cells (cells involved in the immune response) act as the drug. The acronym “CAR” is an abbreviation for “chimeric antigen receptor”; thus, the phrase “CAR-T cell” refers to T cells that have been genetically engineered to express a specific chimeric antigen receptor [1]. An antigen is a small molecule that is recognized by the immune system. Artificial CARs can be designed to target individual surface antigens that are unique to, or overexpressed among, cancer cells such as CD19, CD20, and CD22 [1]. Once the CAR-T cell recognizes a cell expressing the appropriate antigen, it then destroys the target cell [2].
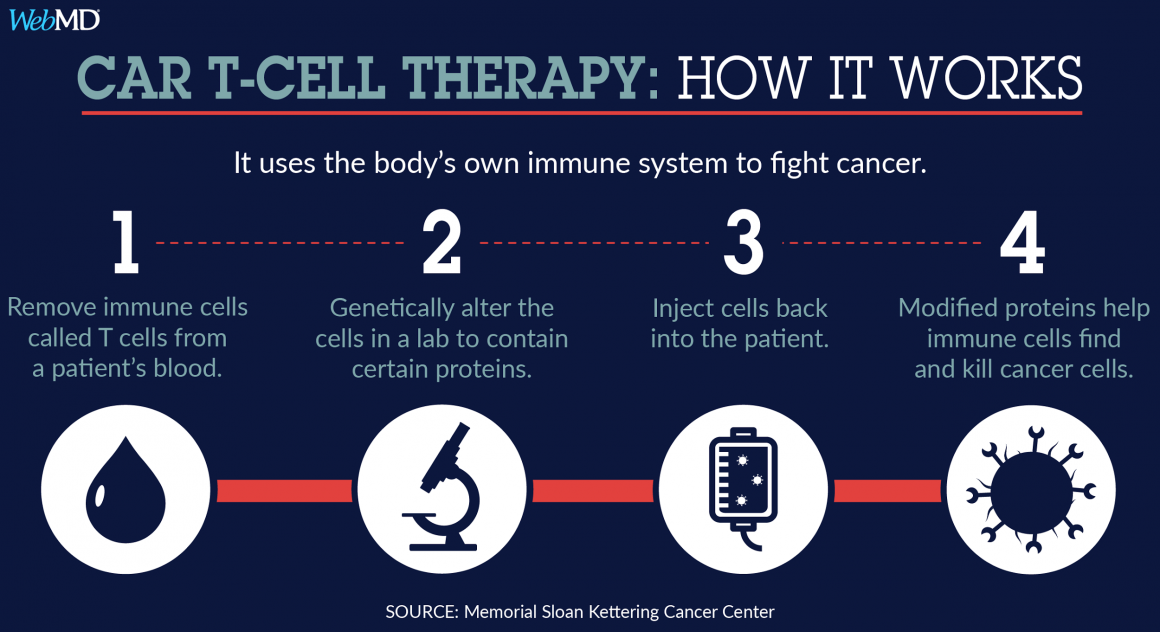
How do researchers transform ordinary T cells into CAR-T cells? The first step is perhaps intuitive: T cells must be isolated from the cancer patient. These T cells must then be concentrated and cultured before introducing a viral vector containing the genetic information for expression of the desired CAR(s) [1,3]. This lentiviral vector introduces RNA encoding the CAR(s) into the T cells that is reverse transcribed into DNA before being integrated into the T cell genome [3]. These genetically modified CAR-T cells are then cultured further and subjected to a series of tests before they are infused into patients [1]. Due to technological advances and improvements to the manufacturing protocol, it only takes 2-4 weeks for the entire process from harvesting the T cells to infusing the CAR-T cells [1].
Current CAR-T cell therapies, including the three FDA approved varieties, primarily target leukemia and lymphoma. Kymriah CAR-T, produced by healthcare company Novartis, targets CD19 antigens that are overexpressed on B cells in the blood cancer acute lymphoblastic leukemia [4,5]. Acute lymphoblastic leukemia is the most prevalent cancer among patients under 20 years old [4]. Existing data on the efficacy of CAR-T cell therapy show much promise as 83% of young adult or juvenile clinical trial patients achieved remission in 3 months or less [4]. Perhaps most importantly, CAR-T cells seem to endure within the patient for an extended period of time following infusion; in one case, a clinical trial patient was found to have CAR-T cells 10 years post-treatment [1].
A cancer treatment that produces high remission rates in a short timespan without toxic chemotherapy sounds like a miracle treatment…but it comes at a price. Novartis, the producer of the Kymriah drug referenced previously, announced that it will charge $475,000 for patients that respond and survive after 1 month of CAR-T cell treatment [4]. This whopping sum – nearly half a million dollars – has the potential to prove financially ruinous to patients who undergo successful treatment. Research is by no means cheap, so it stands to reason that drug companies must put a some sort of a price tag on their products. However, David Mitchell, president of Patients for Affordable Drugs, stated that over $200 million taxpayer dollars contributed to the development of CAR-T cell therapy [4]. In light of this information, and in consideration of the tremendous emotional burden associated with cancer, is it really fair (or even necessary) to charge $475,000 for a life-saving treatment?
Furthermore, even if patients survive and show signs of remission after one month of treatment with CAR-T cell therapy, the therapy is not without side effects. One major side effect that may occur as a result of treatment is cytokine-release syndrome (CRS). CRS is caused by excessive activation of T cells and involves potentially life-threatening symptoms such as inadequate tissue oxygenation, seizures, reduced blood pressure, and fever [5]. This side effect happens more frequently in patients with higher levels of cancer [6]. Another side effect, B-cell aplasia, is more common and is made possible by the fact that CAR-T cell therapy usually targets the CD19 antigen. While this antigen is expressed at significantly elevated levels on cancerous B cells, it is also expressed to a lesser degree on healthy B cells. Thus, CAR-T cells may mistakenly target healthy B cells, impairing the immune system and enhancing the chance of infection [5]. Both of the preceding side effects have the potential to threaten an already fragile patient’s life further and generate additional healthcare costs; thus, the final cost of CAR-T cell treatment may well exceed the already high $475,000 drug price. There is also still a risk that remission will not be permanent as up to one-third of patients experience a relapse within a year of treatment [6]. Relapse is possible even if CAR-T cells endure as cancer cells often stop expressing the target surface antigen, such as CD19, after a certain period of time [6]. A patient might invest over $475,000 only to succumb to the same cancer a year later.
While CAR-T cell therapy for cancer shows promise, it is by no means a perfect solution to the struggle against cancer. Potentially life-threatening side effects and the risk of cancer recurrence cast a long shadow over promising short-term clinical results. Research to circumvent the problem of cancer cell antigen loss is ongoing with some researchers proposing that CAR-T cells be designed to target multiple antigens [6]. Scientists are also exploring the possibility of incorporating “kill switches” in CAR-T cells so that if over-activity occurs and produces adverse side effects the CAR-T cells can be inactivated [1]. However, the most immediate bioethical quandary is the exorbitant cost of CAR-T cell therapy. In an era of heated debate over patient access to affordable healthcare, the cost of lifesaving medical treatment has come under intense scrutiny. Granted, it is not economically feasible for a pharmaceutical company to invest money in the development of a treatment and then offer it for free, but it is also morally reprehensible to charge patients with the price of financial ruin for survival. Some compromise between corporate gain and patient welfare must be achieved to reduce the burden of healthcare costs.
If you are interested in learning more about CAR-T cell therapy and other groundbreaking medical research, consider watching Discovery Channel’s “First In Human” three-part documentary series. It follows the story of several patients at the National Institutes of Health as they go through clinical trials. The series can be found here.
[hr gap=”0″]
Sources:
- Fesnak, A. D., June, C. H., & Levine, B. L. (2016). Engineered T cells: the promise and challenges of cancer immunotherapy. Nature Reviews Cancer,16(9), 566-581. doi:10.1038/nrc.2016.97
- Smith, A. J., Oertle, J., Warren, D., & Prato, D. (2016). Chimeric antigen receptor (CAR) T cell therapy for malignant cancers: Summary and perspective. Journal of Cellular Immunotherapy,2(2), 59-68. doi:10.1016/j.jocit.2016.08.001
- Levine, B. L., Miskin, J., Wonnacott, K., & Keir, C. (2016). Global Manufacturing of CAR T Cell Therapy. Molecular Therapy – Methods & Clinical Development,4, 92-101. doi:10.1016/j.omtm.2016.12.006
- Stein, R. (2017, August 30). FDA Approves First Gene Therapy For Leukemia. Retrieved from https://www.npr.org/sections/health-shots/2017/08/30/547293551/fda-approves-first-gene-therapy-treatment-for-cancer
- Leukemia and Lymphoma Society. (2015, September 10). Chimeric Antigen Receptor (CAR) T-Cell Therapy. Retrieved from https://www.lls.org/treatment/types-of-treatment/immunotherapy/chimeric-antigen-receptor-car-t-cell-therapy
- National Cancer Institute. CAR T Cells: Engineering Immune Cells to Treat Cancer. (2017, August 31). Retrieved from https://www.cancer.gov/about-cancer/treatment/research/car-t-cells