CALLUM ARRAS | BLOGGER | SQ ONLINE (2016-17)
Perhaps those of you who have lived near lakes or estuaries have seen swaths of vibrant green film appear to overtake the natural color of the water. Or maybe you’re one of the lucky few who have been on the beach at Black’s Beach or La Jolla Shores and seen the crashing waves bursting with flashing light as though someone had poured glow sticks into the waves. Does the term “red tide” conjure up thoughts, not of the Alabama juggernaut of football, but a seasonal phenomenon that you may have experienced back at your hometown beach? If any of those scenarios sound familiar to you, then you likely know that, in addition to the striking appearance of these bodies of water during these events, your plans to hang ten or take a dip have been cancelled. Aww, but why?! Well, the pool is full of seemingly inconsiderate microorganisms who have never learned when the party’s over.
Many of you might recognize those trillions of microbes as algae, which come in three exciting flavors: blue-green*, dinoflagellate, and diatomaceous. These microbes are incredibly diverse and contribute to over half of the Earth’s atmospheric oxygen through photosynthesis. They are ever-present in natural water sources and are the all-important first step on the food web, which inevitably ends in bellies of all you sushi fans and spirulina eaters. Like any living population, a particular aquatic habitat can only support a limited number of these algae due to competition for space and resources. Sometimes, conditions occur where the environment can suddenly support the exponential growth of algae in an event ecologists call an algal bloom.
Similarly, the algae, upon rapidly dividing with the new, favorable changes, will inadvertently alter their habitat with profound and often disastrous effects on other organisms in proximity. But why should students at UC San Diego be concerned with these blooming events and what happens to ecosystems that are forced to host an overabundance of these microorganisms? To answer these questions, let me explain how an algal bloom comes about.
*Blue-green algae is a common misnomer for this class of prokaryotic (bacterial) phototrophs. Many taxonomists argue that the term “algae” is reserved exclusively for eukaryotes; therefore, “cyanobacteria” is the preferred identification.
In-Bloom
Autotrophic algae, also known as phytoplankton, are like plants, but smaller and mostly single-celled. Like plants, algae compete for minerals and nutrients that are scarce in their natural environment. As opposed to electrolytes–that’s what plants crave–freshwater algae are essentially limited in their growth rate by a lack of organic phosphorous (yes, of course you know this is an electrolyte), while oceanic algae are limited by a lack of nitrogen (which I am also sure you know is not an electrolyte). If we were to imagine for a moment that a fictional sports-beverage containing these molecules in abundance were spilled into either of the aforementioned habitats where the nutrient concentration were limited, then we would expect an explosion of growth in the benefiting population. This explosion of growth favors phytoplankton more than other aquatic organism, as phytoplankton can more quickly sequester and utilize the nutrients to enhance their generation times. When this happens so rapidly that the organism overwhelms the local environment, we call this occurrence eutrophication, which is Greek for “well-nourished.”

As you may have read in my previous blog regarding El Niño-Southern Oscillation, oceanic temperatures also have a critical influence on the growth of marine fauna; a minute variation of as little as 0.5℃ can precipitate the bleaching of entire coral reefs. Likewise, in freshwater and ocean water, small fluctuations in temperature can disproportionately favor a particular algal species, leading to an overwhelming shift away from the typical population equilibrium and into algal bloom conditions. This advantage would be like observing the students in Geisel on a scorching hot day–we would see the study spaces packed with students in shorts and tees, and hardly any students in jeans and hoodies.
The issue with this explosion of microbes in an environment is that when the microbes die, the dissolved O2 in the environment is rapidly depleted during the decomposition process. The resultant hypoxic (lacking oxygen) conditions contribute to mass dyings of other, larger life forms as they cannot obtain enough oxygen for their own respiration. These blooms last from days to months and can have devastating effects on affected ecosystems, resulting in population collapses and even species extinctions from specific areas. During the Devonian extinction 360 million years ago, between 79% and 87% of all species died out, most of which were aquatic species, which many scientists speculate was caused by consistent and global eutrophication of Earth’s oceans!
It really sucks to HAB blooms
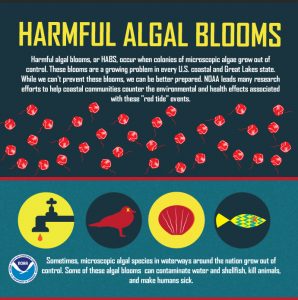
Although dangerous in its own right due to the presence of a decaying biomass, this type of algal bloom is not dangerous to humans since we do not have gills as children or adults and do not need to intake dissolved O2. The real danger to humans in algal blooming are from a variety of toxins released by the algae. Furthermore, most of these toxins must be bioaccumulated in order to become hazardous to human health; according to National Oceanic Atmospheric Administration, directly hazardous toxins, such as microcystins, occur less in less than 1% of algal blooms. When toxins are directly harmful to humans or have the propensity to bioaccumulate, we categorize the event as a harmful algal bloom (HAB).
Take a marine microbiology class with Dr. Brian Palenik or a bacteriology class with Dr. James Golden and you will come to understand just how much influence HABs have on our lives. The Golden lab is looking to better understand microcystins (which cause extensive liver damage in humans) in Anabaena strains, which are known to produce HABs. For one, the toxins produced by these algae are sequestered by other animals, many of which are typical foodstuffs for all sorts of cultures. Just like mercury levels in tuna, ingestion of contaminated fish and shellfish caught proximal to algal blooms is dangerous due to high levels of neurotoxins in these animals. The toxins coming from the algae are robust in water sources and can survive in boiling water. Every year in the U.S., hundreds of people are poisoned and approximately $82 million are lost due to tourism impacts and health care costs.
Dead Zones, Not as Fun as Deadpool
Unsurprisingly, many algal blooms are inadvertently caused by human growth and habitation. Fertilizers and terrestrial organic waste are rich in the macromolecules that marine organisms need for growth. Consequently, when these nutrients travel via runoff or spills to coastlines, streams, and lakes, we invariably see algal blooming. Subsequently many of the developed world’s river outflows experience frequent algal blooming. The consistency of eutrophication in these confluences of chemically-rich water bodies has supported the development of ecological “dead zones” in which nutrient pollution has created seasonal or year-round anoxic environments, unsuitable for marine life. The most famous of these dead zones is the summer Gulf of Mexico ‘dead zone’ which in 2002 was 8,400 sq. miles or larger than the state of Connecticut.

One way in which we can help to prevent HAB’s from occurring locally is to choose cleaning detergents that are marketed as “eco-friendly” and do not contain phosphates or other chemicals that can leech into marine environments. If this is too expensive or your options are limited, consider switching from solid to liquid detergents, or simply using less or it, as concentrations of nitrogen and phosphorus are key to whether or not an algal bloom will occur. Less is more!
[hr gap=”0″]
References and Further Reading:
Scripps Article on HAB – great jumping off point on learning about HABs
Very interesting theory on early Earth extinction
U.S. National Center for Coastal Ocean Science information on HABs
- https://coastalscience.noaa.gov/research/habs/default
- https://products.coastalscience.noaa.gov/pmn/habs.aspx
Live feeds of microbes floating at UCSD’s beaches!
SoCal Coastal Ocean Observing System – good resource for data on HABs